This pipeline computes the correlation between cancer subtypes identified by different molecular patterns and selected clinical features.
Testing the association between subtypes identified by 7 different clustering approaches and 5 clinical features across 430 patients, 30 significant findings detected with P value < 0.05.
-
CNMF clustering analysis on array-based mRNA expression data identified 4 subtypes that correlate to 'AGE', 'HISTOLOGICAL.TYPE', 'RADIATIONS.RADIATION.REGIMENINDICATION', and 'NEOADJUVANT.THERAPY'.
-
Consensus hierarchical clustering analysis on array-based mRNA expression data identified 3 subtypes that correlate to 'HISTOLOGICAL.TYPE' and 'NEOADJUVANT.THERAPY'.
-
3 subtypes identified in current cancer cohort by 'METHLYATION CNMF'. These subtypes correlate to 'Time to Death', 'AGE', 'HISTOLOGICAL.TYPE', 'RADIATIONS.RADIATION.REGIMENINDICATION', and 'NEOADJUVANT.THERAPY'.
-
CNMF clustering analysis on sequencing-based mRNA expression data identified 3 subtypes that correlate to 'Time to Death', 'AGE', 'HISTOLOGICAL.TYPE', 'RADIATIONS.RADIATION.REGIMENINDICATION', and 'NEOADJUVANT.THERAPY'.
-
Consensus hierarchical clustering analysis on sequencing-based mRNA expression data identified 3 subtypes that correlate to 'Time to Death', 'AGE', 'HISTOLOGICAL.TYPE', 'RADIATIONS.RADIATION.REGIMENINDICATION', and 'NEOADJUVANT.THERAPY'.
-
CNMF clustering analysis on sequencing-based miR expression data identified 3 subtypes that correlate to 'Time to Death', 'AGE', 'HISTOLOGICAL.TYPE', and 'NEOADJUVANT.THERAPY'.
-
Consensus hierarchical clustering analysis on sequencing-based miR expression data identified 3 subtypes that correlate to 'Time to Death', 'AGE', 'HISTOLOGICAL.TYPE', 'RADIATIONS.RADIATION.REGIMENINDICATION', and 'NEOADJUVANT.THERAPY'.
Table 1. Get Full Table Overview of the association between subtypes identified by 7 different clustering approaches and 5 clinical features. Shown in the table are P values from statistical tests. Thresholded by P value < 0.05, 30 significant findings detected.
|
Clinical Features |
Time to Death |
AGE |
HISTOLOGICAL TYPE |
RADIATIONS RADIATION REGIMENINDICATION |
NEOADJUVANT THERAPY |
| Statistical Tests | logrank test | ANOVA | Chi-square test | Fisher's exact test | Fisher's exact test |
| mRNA CNMF subtypes | 0.53 | 0.0107 | 0.000456 | 0.0317 | 0.0124 |
| mRNA cHierClus subtypes | 0.73 | 0.0598 | 0.00134 | 0.0666 | 0.0114 |
| METHLYATION CNMF | 0.0061 | 0.0169 | 1.05e-15 | 0.039 | 0.00454 |
| RNAseq CNMF subtypes | 0.00873 | 0.00104 | 8.73e-18 | 0.0148 | 0.000242 |
| RNAseq cHierClus subtypes | 0.00985 | 0.00202 | 8.69e-18 | 0.0325 | 1.45e-05 |
| MIRseq CNMF subtypes | 0.00679 | 4.2e-05 | 9.12e-21 | 0.12 | 0.0066 |
| MIRseq cHierClus subtypes | 0.0098 | 0.000157 | 1.03e-12 | 0.0205 | 0.0221 |
Table S1. Get Full Table Description of clustering approach #1: 'mRNA CNMF subtypes'
| Cluster Labels | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Number of samples | 13 | 19 | 14 | 8 |
P value = 0.53 (logrank test)
Table S2. Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 54 | 7 | 6.0 - 133.2 (35.4) |
| subtype1 | 13 | 2 | 9.0 - 133.2 (39.0) |
| subtype2 | 19 | 3 | 6.0 - 113.2 (37.7) |
| subtype3 | 14 | 1 | 8.6 - 89.3 (30.9) |
| subtype4 | 8 | 1 | 6.4 - 65.5 (22.9) |
Figure S1. Get High-res Image Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #1: 'Time to Death'

P value = 0.0107 (ANOVA)
Table S3. Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 54 | 62.9 (11.8) |
| subtype1 | 13 | 65.1 (12.0) |
| subtype2 | 19 | 68.4 (9.1) |
| subtype3 | 14 | 58.2 (11.0) |
| subtype4 | 8 | 54.8 (12.9) |
Figure S2. Get High-res Image Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #2: 'AGE'

P value = 0.000456 (Chi-square test)
Table S4. Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|
| ALL | 41 | 1 | 12 |
| subtype1 | 12 | 0 | 1 |
| subtype2 | 8 | 0 | 11 |
| subtype3 | 13 | 1 | 0 |
| subtype4 | 8 | 0 | 0 |
Figure S3. Get High-res Image Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.0317 (Fisher's exact test)
Table S5. Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 25 | 29 |
| subtype1 | 7 | 6 |
| subtype2 | 13 | 6 |
| subtype3 | 3 | 11 |
| subtype4 | 2 | 6 |
Figure S4. Get High-res Image Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'

P value = 0.0124 (Fisher's exact test)
Table S6. Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 36 | 18 |
| subtype1 | 7 | 6 |
| subtype2 | 9 | 10 |
| subtype3 | 12 | 2 |
| subtype4 | 8 | 0 |
Figure S5. Get High-res Image Clustering Approach #1: 'mRNA CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'

Table S7. Get Full Table Description of clustering approach #2: 'mRNA cHierClus subtypes'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 20 | 15 | 19 |
P value = 0.73 (logrank test)
Table S8. Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 54 | 7 | 6.0 - 133.2 (35.4) |
| subtype1 | 20 | 2 | 6.4 - 89.3 (29.8) |
| subtype2 | 15 | 2 | 9.0 - 133.2 (39.0) |
| subtype3 | 19 | 3 | 6.0 - 113.2 (37.7) |
Figure S6. Get High-res Image Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'

P value = 0.0598 (ANOVA)
Table S9. Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 54 | 62.9 (11.8) |
| subtype1 | 20 | 58.2 (13.3) |
| subtype2 | 15 | 64.0 (10.6) |
| subtype3 | 19 | 67.1 (9.9) |
Figure S7. Get High-res Image Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #2: 'AGE'
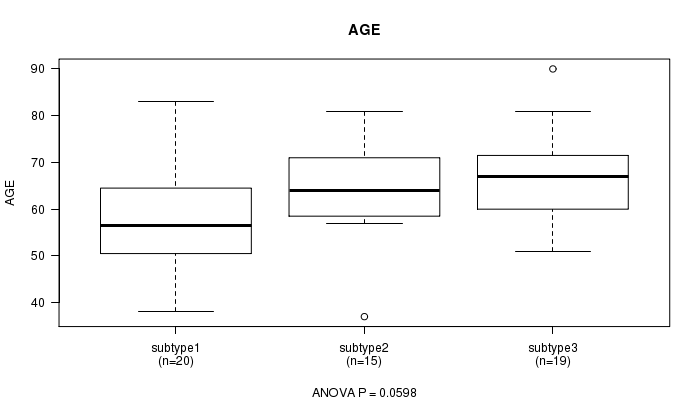
P value = 0.00134 (Chi-square test)
Table S10. Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|
| ALL | 41 | 1 | 12 |
| subtype1 | 19 | 1 | 0 |
| subtype2 | 13 | 0 | 2 |
| subtype3 | 9 | 0 | 10 |
Figure S8. Get High-res Image Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.0666 (Fisher's exact test)
Table S11. Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 25 | 29 |
| subtype1 | 5 | 15 |
| subtype2 | 9 | 6 |
| subtype3 | 11 | 8 |
Figure S9. Get High-res Image Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
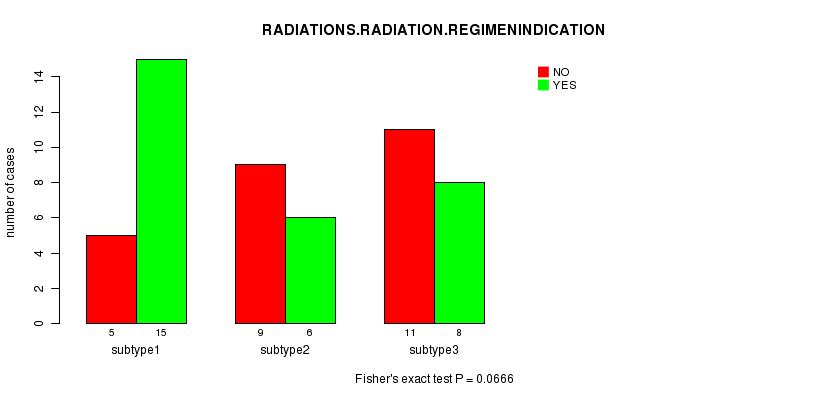
P value = 0.0114 (Fisher's exact test)
Table S12. Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 36 | 18 |
| subtype1 | 18 | 2 |
| subtype2 | 9 | 6 |
| subtype3 | 9 | 10 |
Figure S10. Get High-res Image Clustering Approach #2: 'mRNA cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'

Table S13. Get Full Table Description of clustering approach #3: 'METHLYATION CNMF'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 115 | 76 | 122 |
P value = 0.0061 (logrank test)
Table S14. Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 309 | 30 | 0.0 - 187.1 (12.8) |
| subtype1 | 113 | 18 | 0.0 - 187.1 (11.8) |
| subtype2 | 76 | 4 | 0.0 - 92.0 (16.8) |
| subtype3 | 120 | 8 | 0.3 - 173.6 (12.9) |
Figure S11. Get High-res Image Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #1: 'Time to Death'

P value = 0.0169 (ANOVA)
Table S15. Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 312 | 63.5 (11.1) |
| subtype1 | 114 | 65.6 (9.8) |
| subtype2 | 76 | 63.7 (13.0) |
| subtype3 | 122 | 61.4 (10.7) |
Figure S12. Get High-res Image Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #2: 'AGE'
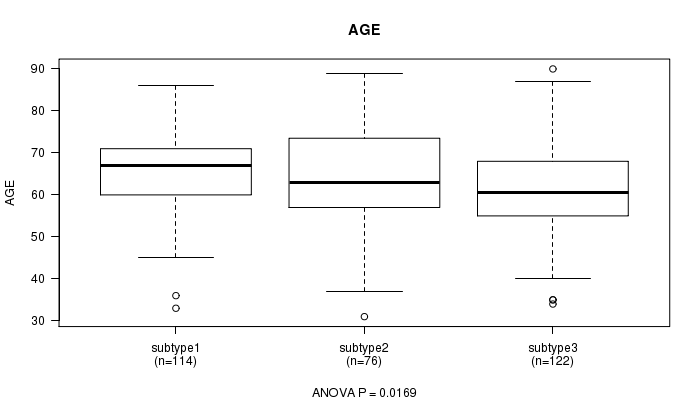
P value = 1.05e-15 (Chi-square test)
Table S16. Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 3) | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|---|---|---|
| ALL | 229 | 6 | 1 | 3 | 16 | 58 |
| subtype1 | 53 | 1 | 0 | 1 | 12 | 48 |
| subtype2 | 60 | 2 | 1 | 0 | 3 | 10 |
| subtype3 | 116 | 3 | 0 | 2 | 1 | 0 |
Figure S13. Get High-res Image Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.039 (Fisher's exact test)
Table S17. Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 79 | 234 |
| subtype1 | 32 | 83 |
| subtype2 | 11 | 65 |
| subtype3 | 36 | 86 |
Figure S14. Get High-res Image Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
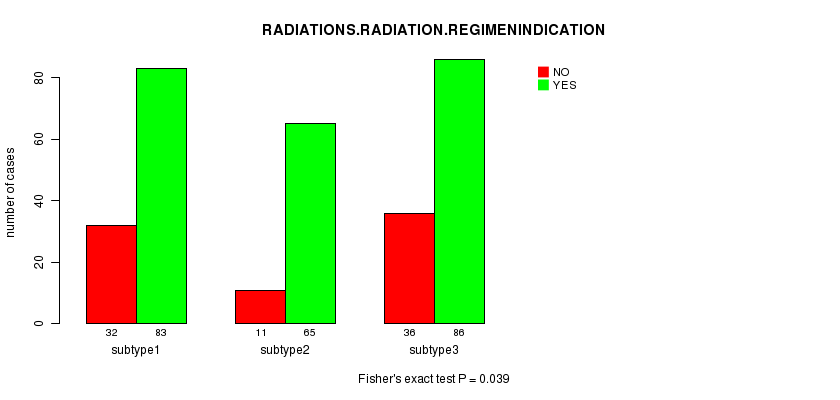
P value = 0.00454 (Fisher's exact test)
Table S18. Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 243 | 70 |
| subtype1 | 78 | 37 |
| subtype2 | 66 | 10 |
| subtype3 | 99 | 23 |
Figure S15. Get High-res Image Clustering Approach #3: 'METHLYATION CNMF' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'

Table S19. Get Full Table Description of clustering approach #4: 'RNAseq CNMF subtypes'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 96 | 83 | 87 |
P value = 0.00873 (logrank test)
Table S20. Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 266 | 20 | 0.0 - 173.6 (20.2) |
| subtype1 | 96 | 13 | 0.5 - 133.2 (17.8) |
| subtype2 | 83 | 2 | 0.6 - 101.1 (21.4) |
| subtype3 | 87 | 5 | 0.0 - 173.6 (22.6) |
Figure S16. Get High-res Image Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #1: 'Time to Death'

P value = 0.00104 (ANOVA)
Table S21. Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 266 | 63.1 (10.8) |
| subtype1 | 96 | 66.2 (10.4) |
| subtype2 | 83 | 62.4 (10.6) |
| subtype3 | 87 | 60.4 (10.7) |
Figure S17. Get High-res Image Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #2: 'AGE'

P value = 8.73e-18 (Chi-square test)
Table S22. Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1 OR 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 3) | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|---|---|---|---|
| ALL | 205 | 3 | 4 | 1 | 5 | 7 | 41 |
| subtype1 | 45 | 0 | 0 | 0 | 4 | 6 | 41 |
| subtype2 | 78 | 2 | 2 | 0 | 0 | 1 | 0 |
| subtype3 | 82 | 1 | 2 | 1 | 1 | 0 | 0 |
Figure S18. Get High-res Image Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.0148 (Fisher's exact test)
Table S23. Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 99 | 167 |
| subtype1 | 44 | 52 |
| subtype2 | 21 | 62 |
| subtype3 | 34 | 53 |
Figure S19. Get High-res Image Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'

P value = 0.000242 (Fisher's exact test)
Table S24. Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 183 | 83 |
| subtype1 | 51 | 45 |
| subtype2 | 64 | 19 |
| subtype3 | 68 | 19 |
Figure S20. Get High-res Image Clustering Approach #4: 'RNAseq CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
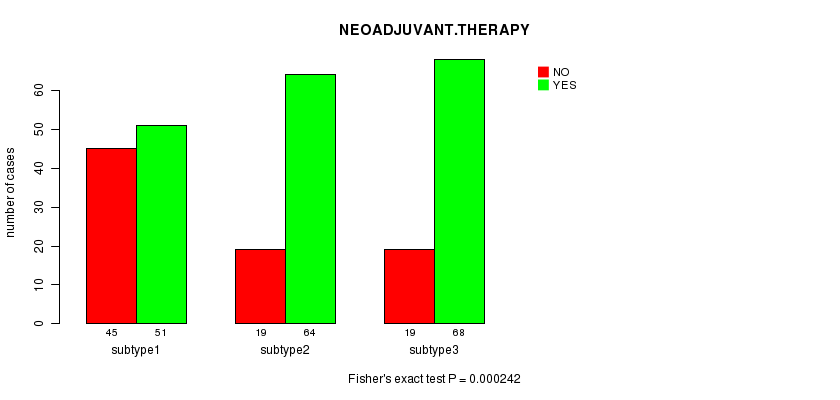
Table S25. Get Full Table Description of clustering approach #5: 'RNAseq cHierClus subtypes'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 96 | 82 | 88 |
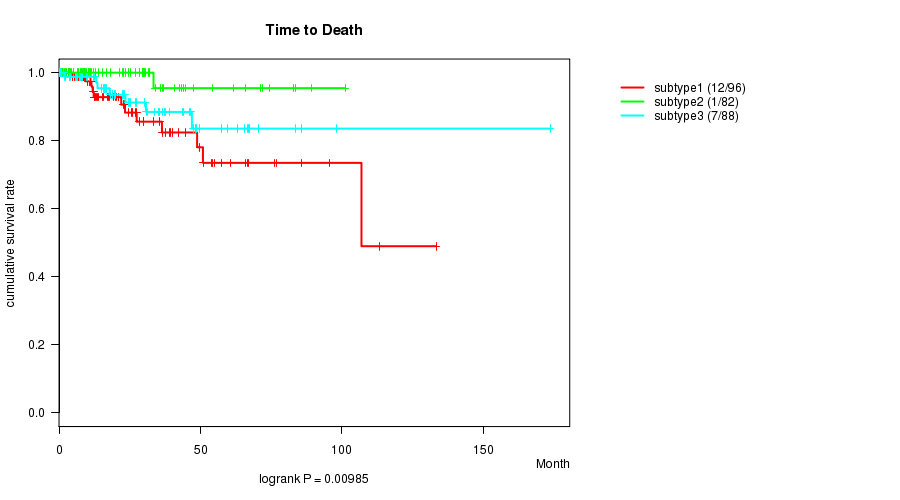
P value = 0.00985 (logrank test)
Table S26. Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 266 | 20 | 0.0 - 173.6 (20.2) |
| subtype1 | 96 | 12 | 0.6 - 133.2 (17.7) |
| subtype2 | 82 | 1 | 0.6 - 101.1 (22.7) |
| subtype3 | 88 | 7 | 0.0 - 173.6 (22.5) |
Figure S21. Get High-res Image Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'
P value = 0.00202 (ANOVA)
Table S27. Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 266 | 63.1 (10.8) |
| subtype1 | 96 | 66.0 (10.4) |
| subtype2 | 82 | 62.5 (10.4) |
| subtype3 | 88 | 60.5 (11.0) |
Figure S22. Get High-res Image Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #2: 'AGE'

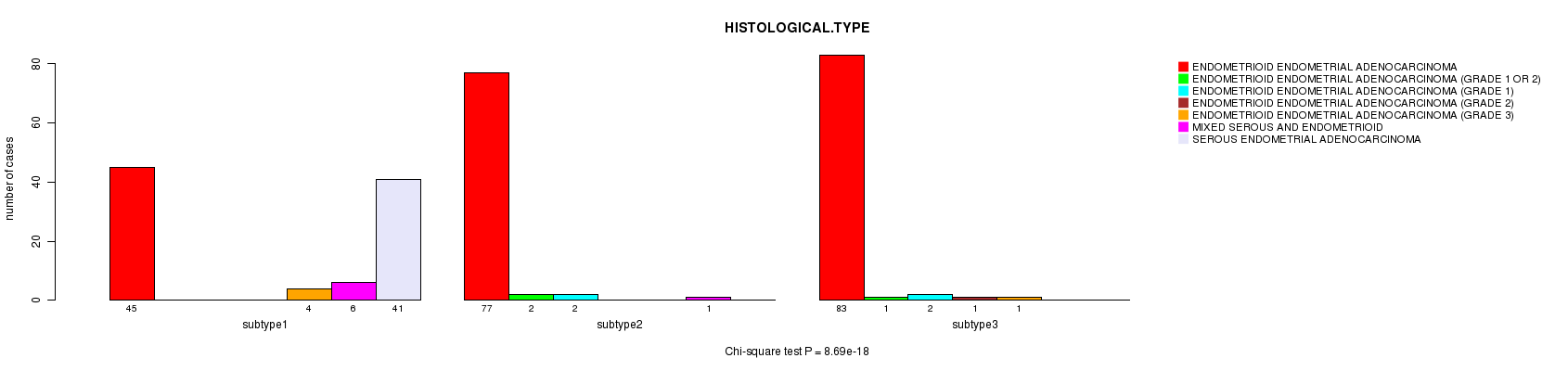
P value = 8.69e-18 (Chi-square test)
Table S28. Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1 OR 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 3) | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|---|---|---|---|
| ALL | 205 | 3 | 4 | 1 | 5 | 7 | 41 |
| subtype1 | 45 | 0 | 0 | 0 | 4 | 6 | 41 |
| subtype2 | 77 | 2 | 2 | 0 | 0 | 1 | 0 |
| subtype3 | 83 | 1 | 2 | 1 | 1 | 0 | 0 |
Figure S23. Get High-res Image Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
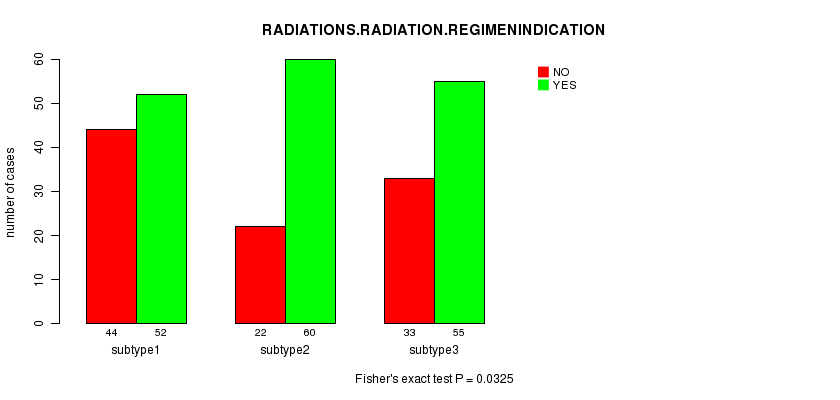
P value = 0.0325 (Fisher's exact test)
Table S29. Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 99 | 167 |
| subtype1 | 44 | 52 |
| subtype2 | 22 | 60 |
| subtype3 | 33 | 55 |
Figure S24. Get High-res Image Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
P value = 1.45e-05 (Fisher's exact test)
Table S30. Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 183 | 83 |
| subtype1 | 49 | 47 |
| subtype2 | 67 | 15 |
| subtype3 | 67 | 21 |
Figure S25. Get High-res Image Clustering Approach #5: 'RNAseq cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'

Table S31. Get Full Table Description of clustering approach #6: 'MIRseq CNMF subtypes'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 141 | 125 | 100 |
P value = 0.00679 (logrank test)
Table S32. Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 364 | 29 | 0.0 - 187.1 (17.4) |
| subtype1 | 140 | 17 | 0.0 - 187.1 (15.8) |
| subtype2 | 124 | 3 | 0.1 - 101.1 (17.4) |
| subtype3 | 100 | 9 | 0.5 - 173.6 (23.3) |
Figure S26. Get High-res Image Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #1: 'Time to Death'

P value = 4.2e-05 (ANOVA)
Table S33. Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 366 | 63.1 (11.2) |
| subtype1 | 141 | 66.0 (10.7) |
| subtype2 | 125 | 62.8 (11.6) |
| subtype3 | 100 | 59.5 (10.4) |
Figure S27. Get High-res Image Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #2: 'AGE'
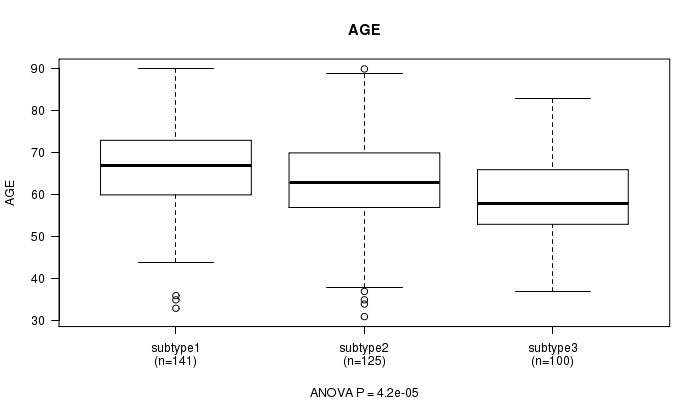
P value = 9.12e-21 (Chi-square test)
Table S34. Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1 OR 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 3) | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|---|---|---|---|
| ALL | 281 | 3 | 9 | 2 | 7 | 13 | 51 |
| subtype1 | 74 | 0 | 1 | 0 | 4 | 11 | 51 |
| subtype2 | 118 | 2 | 3 | 0 | 1 | 1 | 0 |
| subtype3 | 89 | 1 | 5 | 2 | 2 | 1 | 0 |
Figure S28. Get High-res Image Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.12 (Fisher's exact test)
Table S35. Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 121 | 245 |
| subtype1 | 55 | 86 |
| subtype2 | 34 | 91 |
| subtype3 | 32 | 68 |
Figure S29. Get High-res Image Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'

P value = 0.0066 (Fisher's exact test)
Table S36. Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 272 | 94 |
| subtype1 | 92 | 49 |
| subtype2 | 102 | 23 |
| subtype3 | 78 | 22 |
Figure S30. Get High-res Image Clustering Approach #6: 'MIRseq CNMF subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
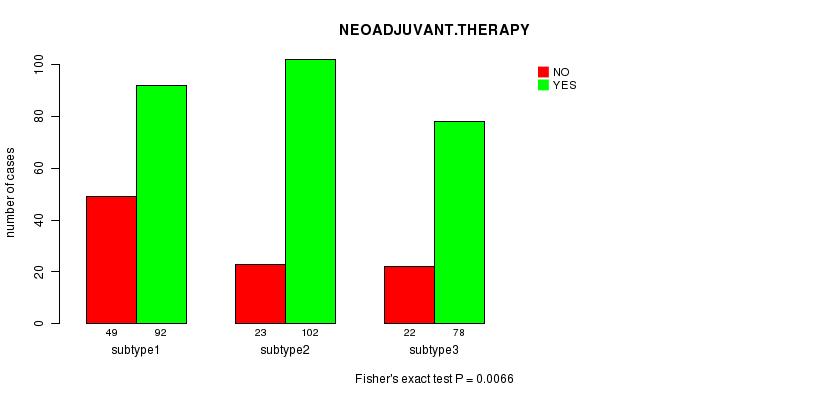
Table S37. Get Full Table Description of clustering approach #7: 'MIRseq cHierClus subtypes'
| Cluster Labels | 1 | 2 | 3 |
|---|---|---|---|
| Number of samples | 112 | 177 | 77 |
P value = 0.0098 (logrank test)
Table S38. Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 364 | 29 | 0.0 - 187.1 (17.4) |
| subtype1 | 112 | 2 | 0.1 - 101.1 (16.6) |
| subtype2 | 175 | 18 | 0.0 - 187.1 (17.7) |
| subtype3 | 77 | 9 | 0.7 - 173.6 (18.2) |
Figure S31. Get High-res Image Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #1: 'Time to Death'

P value = 0.000157 (ANOVA)
Table S39. Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #2: 'AGE'
| nPatients | Mean (Std.Dev) | |
|---|---|---|
| ALL | 366 | 63.1 (11.2) |
| subtype1 | 112 | 63.4 (10.4) |
| subtype2 | 177 | 64.9 (11.1) |
| subtype3 | 77 | 58.6 (11.5) |
Figure S32. Get High-res Image Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #2: 'AGE'

P value = 1.03e-12 (Chi-square test)
Table S40. Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'
| nPatients | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1 OR 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 1) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 2) | ENDOMETRIOID ENDOMETRIAL ADENOCARCINOMA (GRADE 3) | MIXED SEROUS AND ENDOMETRIOID | SEROUS ENDOMETRIAL ADENOCARCINOMA |
|---|---|---|---|---|---|---|---|
| ALL | 281 | 3 | 9 | 2 | 7 | 13 | 51 |
| subtype1 | 103 | 2 | 4 | 0 | 0 | 1 | 2 |
| subtype2 | 110 | 0 | 2 | 0 | 4 | 12 | 49 |
| subtype3 | 68 | 1 | 3 | 2 | 3 | 0 | 0 |
Figure S33. Get High-res Image Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #3: 'HISTOLOGICAL.TYPE'

P value = 0.0205 (Fisher's exact test)
Table S41. Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
| nPatients | NO | YES |
|---|---|---|
| ALL | 121 | 245 |
| subtype1 | 31 | 81 |
| subtype2 | 71 | 106 |
| subtype3 | 19 | 58 |
Figure S34. Get High-res Image Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #4: 'RADIATIONS.RADIATION.REGIMENINDICATION'
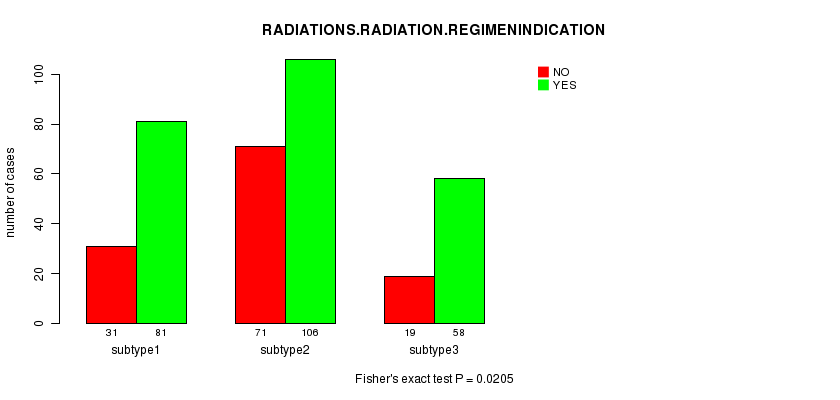
P value = 0.0221 (Fisher's exact test)
Table S42. Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'
| nPatients | NO | YES |
|---|---|---|
| ALL | 272 | 94 |
| subtype1 | 89 | 23 |
| subtype2 | 120 | 57 |
| subtype3 | 63 | 14 |
Figure S35. Get High-res Image Clustering Approach #7: 'MIRseq cHierClus subtypes' versus Clinical Feature #5: 'NEOADJUVANT.THERAPY'

-
Cluster data file = UCEC.mergedcluster.txt
-
Clinical data file = UCEC.clin.merged.picked.txt
-
Number of patients = 430
-
Number of clustering approaches = 7
-
Number of selected clinical features = 5
-
Exclude small clusters that include fewer than K patients, K = 3
consensus non-negative matrix factorization clustering approach (Brunet et al. 2004)
Resampling-based clustering method (Monti et al. 2003)
For survival clinical features, the Kaplan-Meier survival curves of tumors with and without gene mutations were plotted and the statistical significance P values were estimated by logrank test (Bland and Altman 2004) using the 'survdiff' function in R
For continuous numerical clinical features, one-way analysis of variance (Howell 2002) was applied to compare the clinical values between tumor subtypes using 'anova' function in R
For multi-class clinical features (nominal or ordinal), Chi-square tests (Greenwood and Nikulin 1996) were used to estimate the P values using the 'chisq.test' function in R
For binary clinical features, two-tailed Fisher's exact tests (Fisher 1922) were used to estimate the P values using the 'fisher.test' function in R
This is an experimental feature. The full results of the analysis summarized in this report can be downloaded from the TCGA Data Coordination Center.