This pipeline computes the correlation between significant arm-level copy number variations (cnvs) and selected clinical features.
Testing the association between subtypes identified by 22 different clustering approaches and 3 clinical features across 191 patients, 4 significant findings detected with Q value < 0.25.
-
2 subtypes identified in current cancer cohort by '1p gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '4p gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '4q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '8p gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '8q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '10q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '11p gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '11q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '13q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '17q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '19p gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '19q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '21q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '22q gain mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '5q loss mutation analysis'. These subtypes correlate to 'Time to Death'.
-
2 subtypes identified in current cancer cohort by '7p loss mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '7q loss mutation analysis'. These subtypes correlate to 'Time to Death'.
-
2 subtypes identified in current cancer cohort by '12p loss mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '17p loss mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '17q loss mutation analysis'. These subtypes do not correlate to any clinical features.
-
2 subtypes identified in current cancer cohort by '18p loss mutation analysis'. These subtypes correlate to 'Time to Death'.
-
2 subtypes identified in current cancer cohort by '18q loss mutation analysis'. These subtypes correlate to 'Time to Death'.
Table 1. Get Full Table Overview of the association between subtypes identified by 22 different clustering approaches and 3 clinical features. Shown in the table are P values (Q values). Thresholded by Q value < 0.25, 4 significant findings detected.
|
Clinical Features |
Time to Death |
AGE | GENDER |
| Statistical Tests | logrank test | t-test | Fisher's exact test |
| 1p gain |
0.0913 (1.00) |
0.592 (1.00) |
|
| 4p gain |
0.587 (1.00) |
0.425 (1.00) |
0.627 (1.00) |
| 4q gain |
0.587 (1.00) |
0.425 (1.00) |
0.627 (1.00) |
| 8p gain |
0.48 (1.00) |
0.119 (1.00) |
0.0597 (1.00) |
| 8q gain |
0.529 (1.00) |
0.168 (1.00) |
0.0382 (1.00) |
| 10q gain |
0.947 (1.00) |
0.252 (1.00) |
|
| 11p gain |
0.188 (1.00) |
1 (1.00) |
|
| 11q gain |
0.57 (1.00) |
0.0154 (0.834) |
1 (1.00) |
| 13q gain |
0.511 (1.00) |
0.592 (1.00) |
|
| 17q gain |
0.729 (1.00) |
0.29 (1.00) |
1 (1.00) |
| 19p gain |
0.347 (1.00) |
0.789 (1.00) |
0.0642 (1.00) |
| 19q gain |
0.347 (1.00) |
0.789 (1.00) |
0.0642 (1.00) |
| 21q gain |
0.0186 (0.966) |
0.616 (1.00) |
0.0732 (1.00) |
| 22q gain |
0.887 (1.00) |
0.00598 (0.341) |
0.73 (1.00) |
| 5q loss |
0.00073 (0.0438) |
0.0601 (1.00) |
0.0325 (1.00) |
| 7p loss |
0.00716 (0.401) |
0.325 (1.00) |
1 (1.00) |
| 7q loss |
0.00315 (0.183) |
0.325 (1.00) |
0.805 (1.00) |
| 12p loss |
0.514 (1.00) |
1 (1.00) |
|
| 17p loss |
0.376 (1.00) |
0.308 (1.00) |
0.114 (1.00) |
| 17q loss |
0.0166 (0.882) |
0.0075 (0.412) |
0.378 (1.00) |
| 18p loss |
0.00202 (0.119) |
0.355 (1.00) |
0.0642 (1.00) |
| 18q loss |
0.000113 (0.00689) |
0.0297 (1.00) |
0.252 (1.00) |
Table S1. Get Full Table Description of clustering approach #1: '1p gain mutation analysis'
| Cluster Labels | 1P GAIN MUTATED | 1P GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
Table S2. Get Full Table Description of clustering approach #2: '4p gain mutation analysis'
| Cluster Labels | 4P GAIN MUTATED | 4P GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 4 | 187 |
Table S3. Get Full Table Description of clustering approach #3: '4q gain mutation analysis'
| Cluster Labels | 4Q GAIN MUTATED | 4Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 4 | 187 |
Table S4. Get Full Table Description of clustering approach #4: '8p gain mutation analysis'
| Cluster Labels | 8P GAIN MUTATED | 8P GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 20 | 171 |
Table S5. Get Full Table Description of clustering approach #5: '8q gain mutation analysis'
| Cluster Labels | 8Q GAIN MUTATED | 8Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 21 | 170 |
Table S6. Get Full Table Description of clustering approach #6: '10q gain mutation analysis'
| Cluster Labels | 10Q GAIN MUTATED | 10Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
Table S7. Get Full Table Description of clustering approach #7: '11p gain mutation analysis'
| Cluster Labels | 11P GAIN MUTATED | 11P GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 4 | 187 |
Table S8. Get Full Table Description of clustering approach #8: '11q gain mutation analysis'
| Cluster Labels | 11Q GAIN MUTATED | 11Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 7 | 184 |
Table S9. Get Full Table Description of clustering approach #9: '13q gain mutation analysis'
| Cluster Labels | 13Q GAIN MUTATED | 13Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
Table S10. Get Full Table Description of clustering approach #10: '17q gain mutation analysis'
| Cluster Labels | 17Q GAIN MUTATED | 17Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
Table S11. Get Full Table Description of clustering approach #11: '19p gain mutation analysis'
| Cluster Labels | 19P GAIN MUTATED | 19P GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 5 | 186 |
Table S12. Get Full Table Description of clustering approach #12: '19q gain mutation analysis'
| Cluster Labels | 19Q GAIN MUTATED | 19Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 5 | 186 |
Table S13. Get Full Table Description of clustering approach #13: '21q gain mutation analysis'
| Cluster Labels | 21Q GAIN MUTATED | 21Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 8 | 183 |
Table S14. Get Full Table Description of clustering approach #14: '22q gain mutation analysis'
| Cluster Labels | 22Q GAIN MUTATED | 22Q GAIN WILD-TYPE |
|---|---|---|
| Number of samples | 8 | 183 |
Table S15. Get Full Table Description of clustering approach #15: '5q loss mutation analysis'
| Cluster Labels | 5Q LOSS MUTATED | 5Q LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 6 | 185 |
P value = 0.00073 (logrank test), Q value = 0.044
Table S16. Clustering Approach #15: '5q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 168 | 106 | 0.9 - 94.1 (12.0) |
| 5Q LOSS MUTATED | 6 | 6 | 1.0 - 12.0 (7.0) |
| 5Q LOSS WILD-TYPE | 162 | 100 | 0.9 - 94.1 (12.5) |
Figure S1. Get High-res Image Clustering Approach #15: '5q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
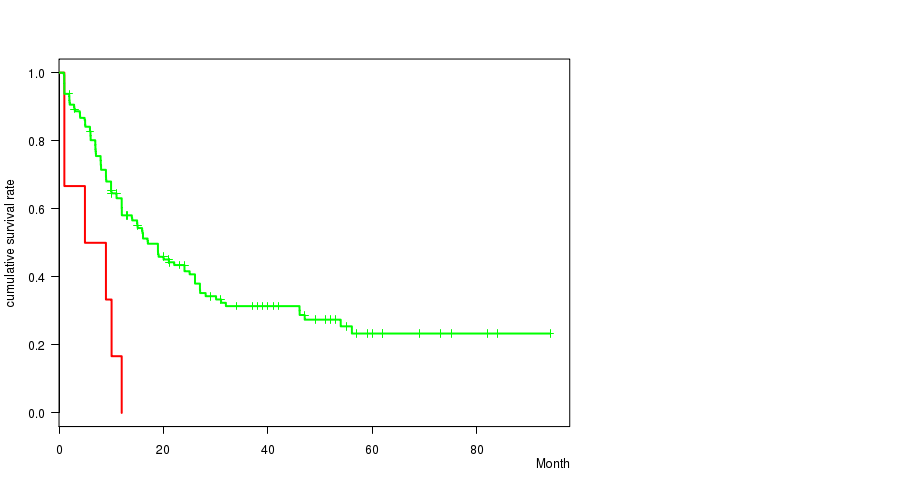
Table S17. Get Full Table Description of clustering approach #16: '7p loss mutation analysis'
| Cluster Labels | 7P LOSS MUTATED | 7P LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 15 | 176 |
Table S18. Get Full Table Description of clustering approach #17: '7q loss mutation analysis'
| Cluster Labels | 7Q LOSS MUTATED | 7Q LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 18 | 173 |
P value = 0.00315 (logrank test), Q value = 0.18
Table S19. Clustering Approach #17: '7q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 168 | 106 | 0.9 - 94.1 (12.0) |
| 7Q LOSS MUTATED | 15 | 13 | 1.0 - 40.0 (9.0) |
| 7Q LOSS WILD-TYPE | 153 | 93 | 0.9 - 94.1 (13.9) |
Figure S2. Get High-res Image Clustering Approach #17: '7q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
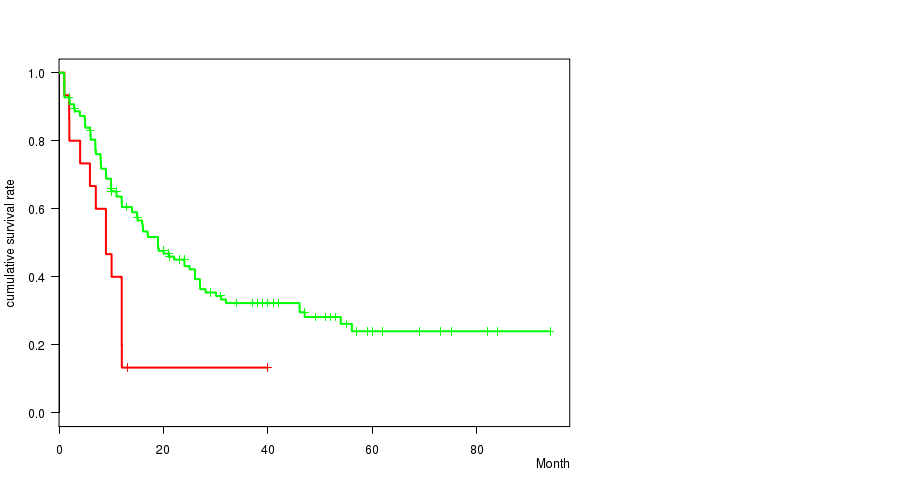
Table S20. Get Full Table Description of clustering approach #18: '12p loss mutation analysis'
| Cluster Labels | 12P LOSS MUTATED | 12P LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
Table S21. Get Full Table Description of clustering approach #19: '17p loss mutation analysis'
| Cluster Labels | 17P LOSS MUTATED | 17P LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 10 | 181 |
Table S22. Get Full Table Description of clustering approach #20: '17q loss mutation analysis'
| Cluster Labels | 17Q LOSS MUTATED | 17Q LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 5 | 186 |
Table S23. Get Full Table Description of clustering approach #21: '18p loss mutation analysis'
| Cluster Labels | 18P LOSS MUTATED | 18P LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 5 | 186 |
P value = 0.00202 (logrank test), Q value = 0.12
Table S24. Clustering Approach #21: '18p loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 168 | 106 | 0.9 - 94.1 (12.0) |
| 18P LOSS MUTATED | 5 | 5 | 1.0 - 12.0 (7.0) |
| 18P LOSS WILD-TYPE | 163 | 101 | 0.9 - 94.1 (12.0) |
Figure S3. Get High-res Image Clustering Approach #21: '18p loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
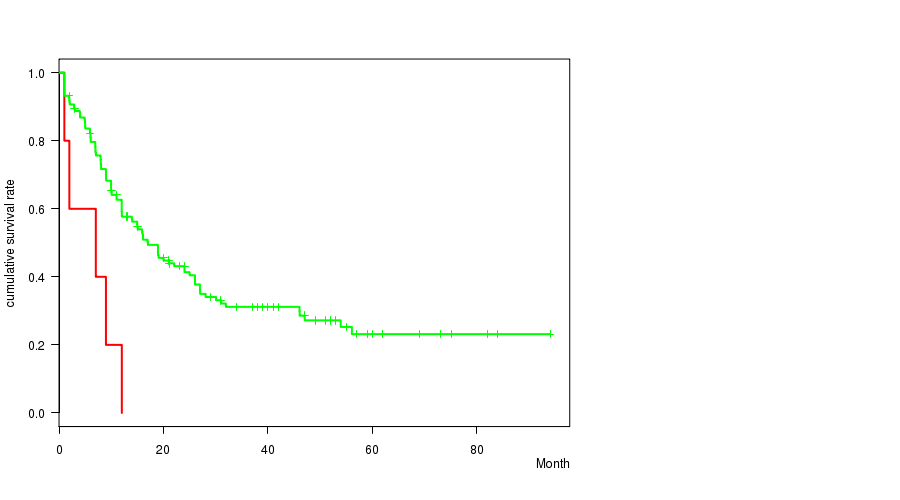
Table S25. Get Full Table Description of clustering approach #22: '18q loss mutation analysis'
| Cluster Labels | 18Q LOSS MUTATED | 18Q LOSS WILD-TYPE |
|---|---|---|
| Number of samples | 3 | 188 |
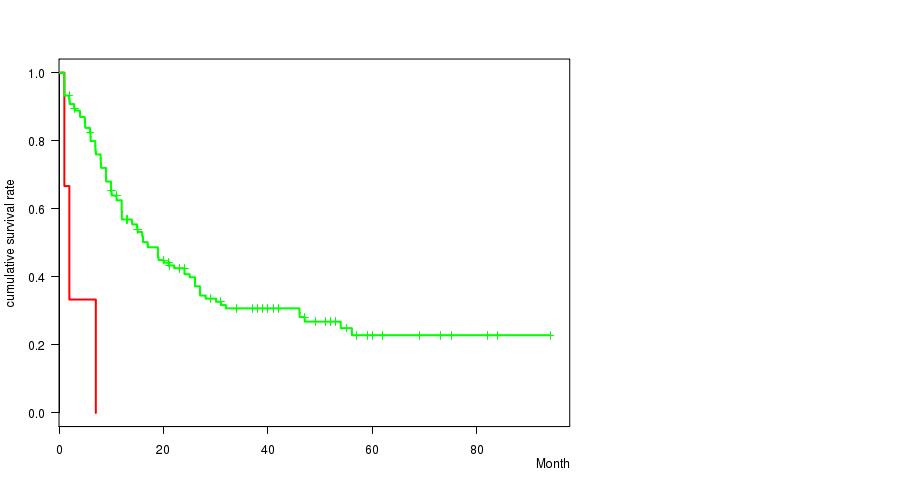
P value = 0.000113 (logrank test), Q value = 0.0069
Table S26. Clustering Approach #22: '18q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
| nPatients | nDeath | Duration Range (Median), Month | |
|---|---|---|---|
| ALL | 168 | 106 | 0.9 - 94.1 (12.0) |
| 18Q LOSS MUTATED | 3 | 3 | 1.0 - 7.0 (2.0) |
| 18Q LOSS WILD-TYPE | 165 | 103 | 0.9 - 94.1 (12.0) |
Figure S4. Get High-res Image Clustering Approach #22: '18q loss mutation analysis' versus Clinical Feature #1: 'Time to Death'
-
Cluster data file = broad_values_by_arm.mutsig.cluster.txt
-
Clinical data file = LAML-TB.clin.merged.picked.txt
-
Number of patients = 191
-
Number of clustering approaches = 22
-
Number of selected clinical features = 3
-
Exclude small clusters that include fewer than K patients, K = 3
For survival clinical features, the Kaplan-Meier survival curves of tumors with and without gene mutations were plotted and the statistical significance P values were estimated by logrank test (Bland and Altman 2004) using the 'survdiff' function in R
For continuous numerical clinical features, two-tailed Student's t test with unequal variance (Lehmann and Romano 2005) was applied to compare the clinical values between two tumor subtypes using 't.test' function in R
For binary clinical features, two-tailed Fisher's exact tests (Fisher 1922) were used to estimate the P values using the 'fisher.test' function in R
For multiple hypothesis correction, Q value is the False Discovery Rate (FDR) analogue of the P value (Benjamini and Hochberg 1995), defined as the minimum FDR at which the test may be called significant. We used the 'Benjamini and Hochberg' method of 'p.adjust' function in R to convert P values into Q values.
This is an experimental feature. The full results of the analysis summarized in this report can be downloaded from the TCGA Data Coordination Center.