This report serves to describe the mutational landscape and properties of a given individual set, as well as rank genes and genesets according to mutational significance. MutSig v2.0 and MutSigCV v0.9 merged result was used to generate the results found in this report.
-
Working with individual set: KIRP-TP
-
Number of patients in set: 111
The input for this pipeline is a set of individuals with the following files associated for each:
-
An annotated .maf file describing the mutations called for the respective individual, and their properties.
-
A .wig file that contains information about the coverage of the sample.
-
MAF used for this analysis:KIRP-TP.final_analysis_set.maf
-
Significantly mutated genes (q ≤ 0.1): 5
-
Mutations seen in COSMIC: 43
-
Significantly mutated genes in COSMIC territory: 11
-
Significantly mutated genesets: 0
-
Read 111 MAFs of type "Broad"
-
Total number of mutations in input MAFs: 10131
-
After removing 42 mutations outside chr1-24: 10089
-
After removing 348 blacklisted mutations: 9741
-
After removing 259 noncoding mutations: 9482
-
After collapsing adjacent/redundant mutations: 8036
-
Number of mutations before filtering: 8036
-
After removing 124 mutations outside gene set: 7912
-
After removing 5 mutations outside category set: 7907
Table 1. Get Full Table Table representing breakdown of mutations by type.
| type | count |
|---|---|
| Frame_Shift_Del | 528 |
| Frame_Shift_Ins | 201 |
| In_Frame_Del | 131 |
| In_Frame_Ins | 29 |
| Missense_Mutation | 4829 |
| Nonsense_Mutation | 266 |
| Nonstop_Mutation | 6 |
| Silent | 1725 |
| Splice_Site | 178 |
| Translation_Start_Site | 14 |
| Total | 7907 |
Table 2. Get Full Table A breakdown of mutation rates per category discovered for this individual set.
| category | n | N | rate | rate_per_mb | relative_rate | exp_ns_s_ratio |
|---|---|---|---|---|---|---|
| *CpG->T | 511 | 184714396 | 2.8e-06 | 2.8 | 1.5 | 2.1 |
| *Cp(A/C/T)->T | 905 | 1496190153 | 6e-07 | 0.6 | 0.32 | 1.7 |
| A->G | 894 | 1608163559 | 5.6e-07 | 0.56 | 0.3 | 2.3 |
| transver | 2532 | 3289068108 | 7.7e-07 | 0.77 | 0.41 | 5 |
| indel+null | 1335 | 3289068108 | 4.1e-07 | 0.41 | 0.22 | NaN |
| double_null | 5 | 3289068108 | 1.5e-09 | 0.0015 | 0.00081 | NaN |
| Total | 6182 | 3289068108 | 1.9e-06 | 1.9 | 1 | 3.5 |
The x axis represents the samples. The y axis represents the exons, one row per exon, and they are sorted by average coverage across samples. For exons with exactly the same average coverage, they are sorted next by the %GC of the exon. (The secondary sort is especially useful for the zero-coverage exons at the bottom).
Figure 1.
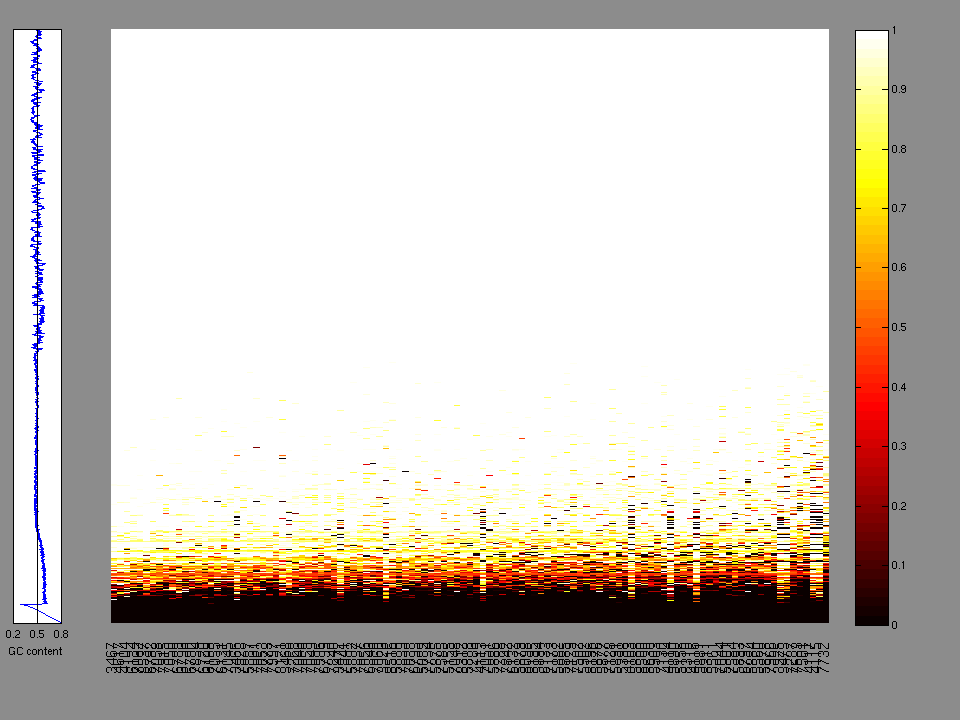
Figure 2. Patients counts and rates file used to generate this plot: KIRP-TP.patients.counts_and_rates.txt
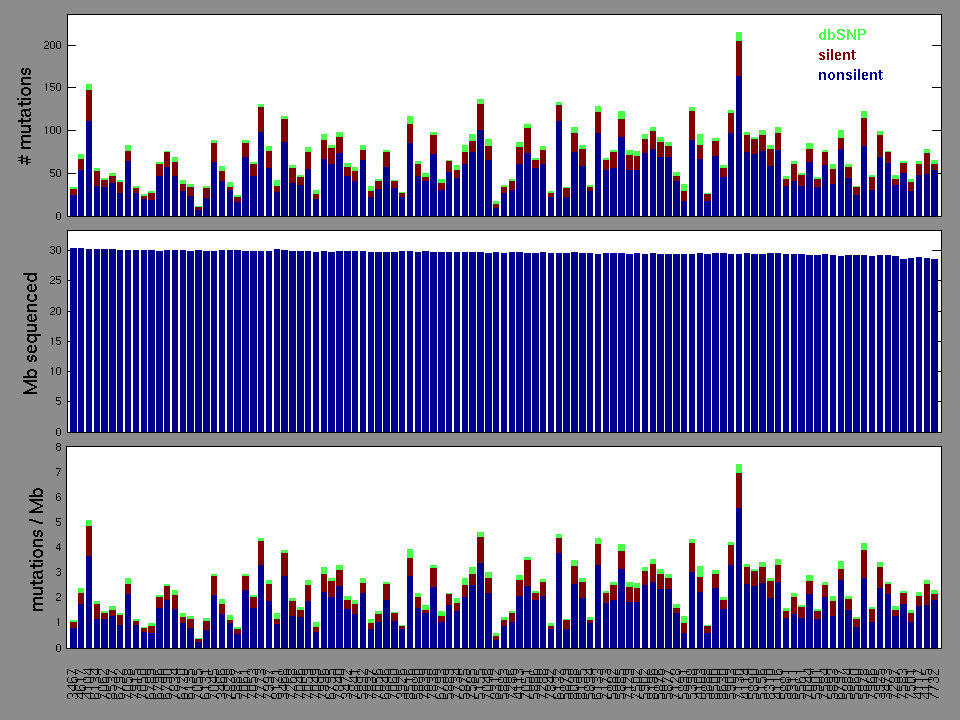
Figure 3. Needs description.
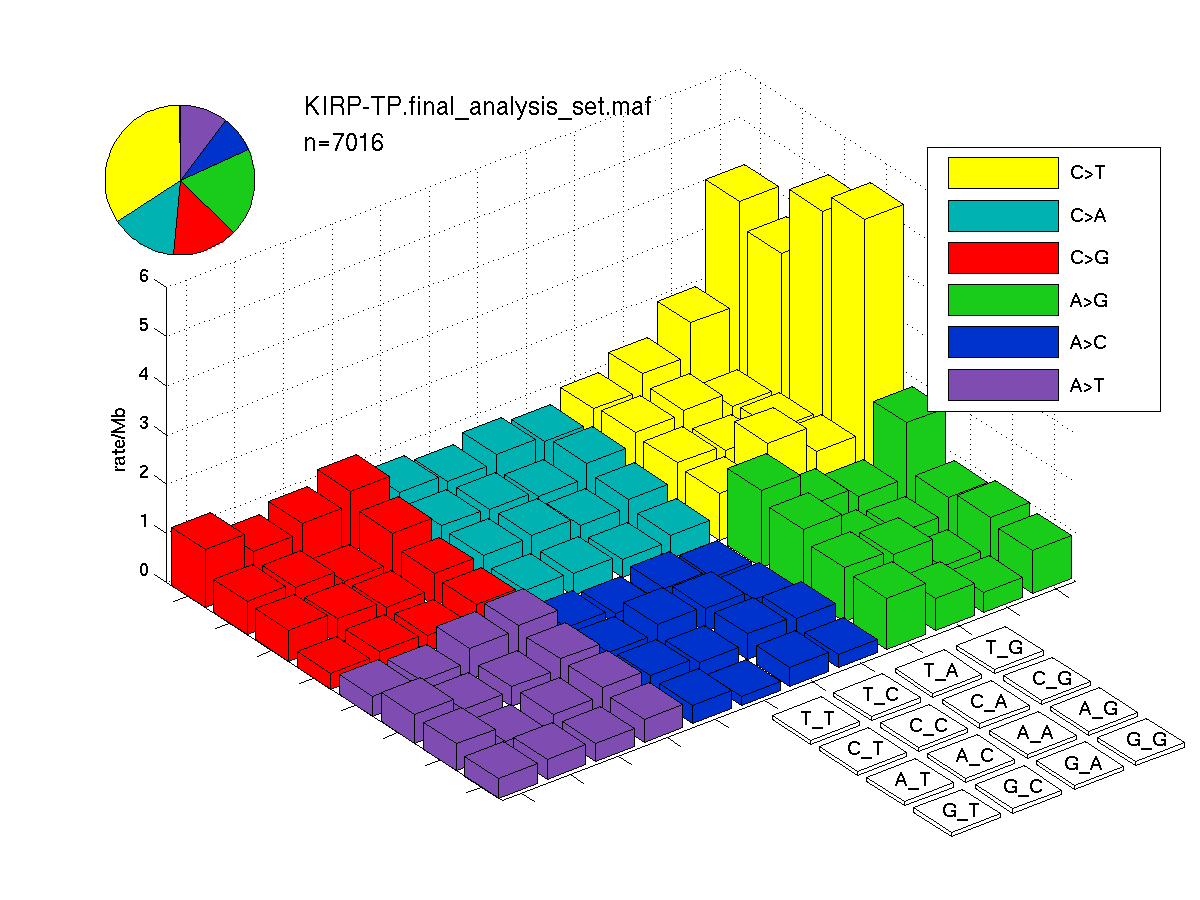
Figure 4. Needs description.
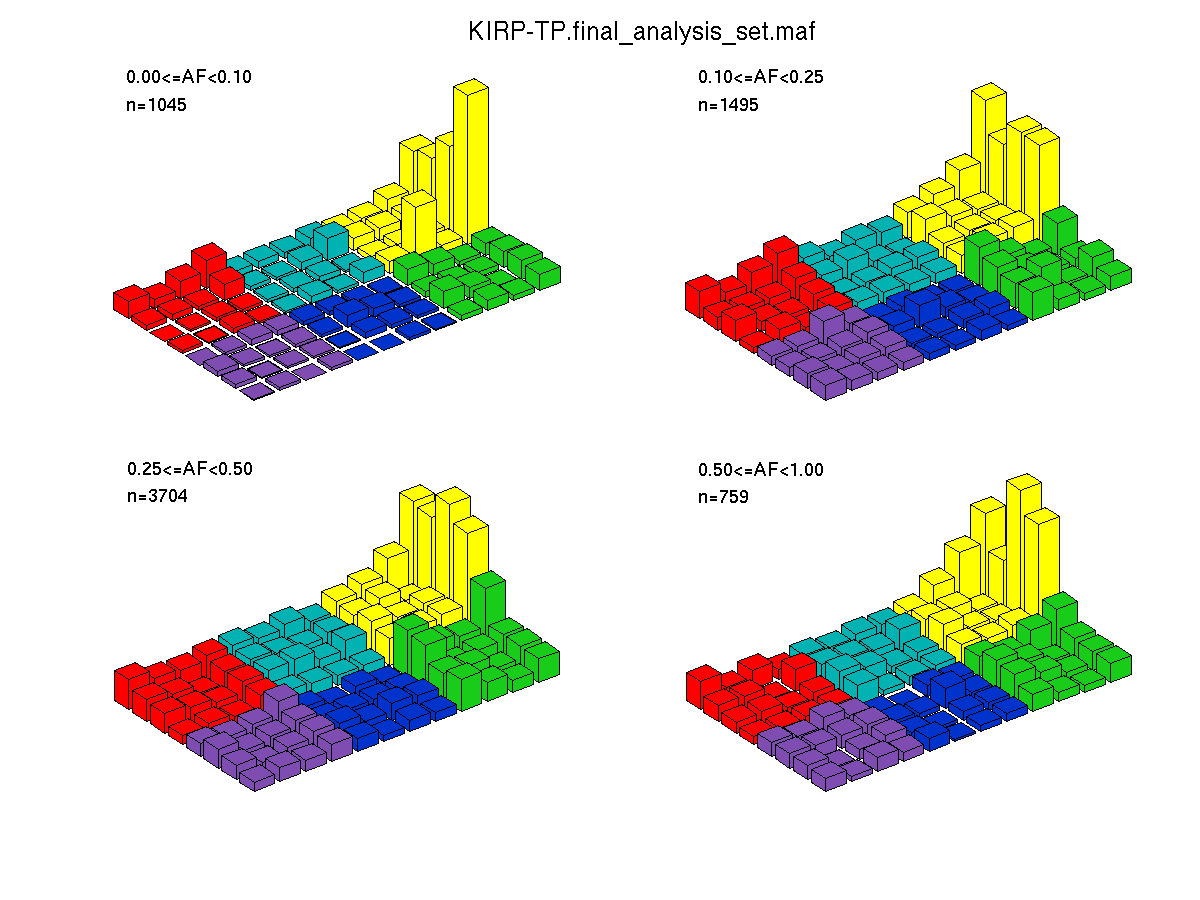
Figure 5. Get High-res Image The matrix in the center of the figure represents individual mutations in patient samples, color-coded by type of mutation, for the significantly mutated genes. The rate of synonymous and non-synonymous mutations is displayed at the top of the matrix. The barplot on the left of the matrix shows the number of mutations in each gene. The percentages represent the fraction of tumors with at least one mutation in the specified gene. The barplot to the right of the matrix displays the q-values for the most significantly mutated genes. The purple boxplots below the matrix (only displayed if required columns are present in the provided MAF) represent the distributions of allelic fractions observed in each sample. The plot at the bottom represents the base substitution distribution of individual samples, using the same categories that were used to calculate significance.
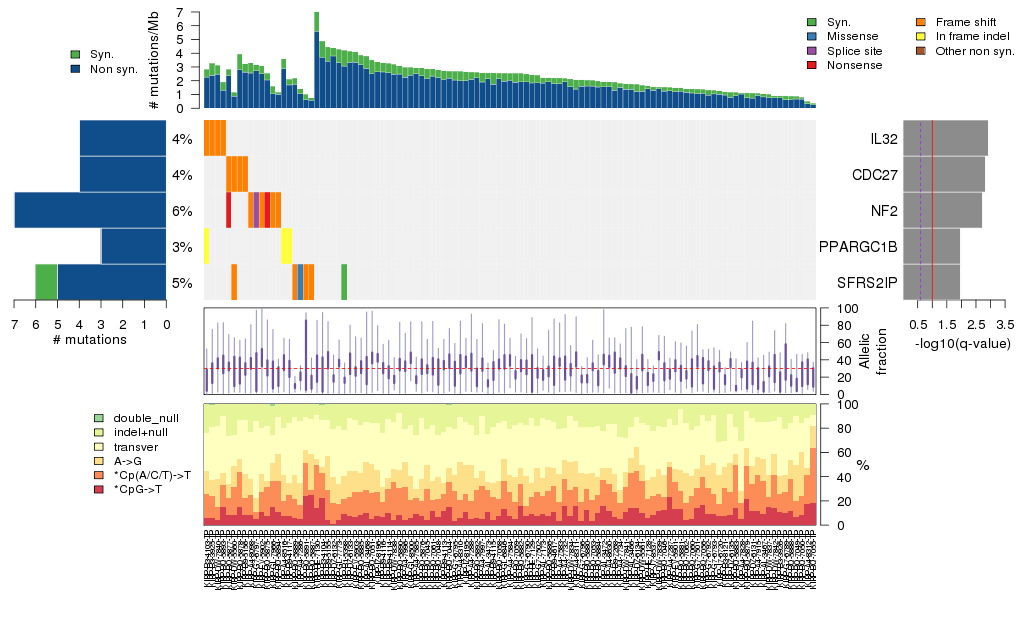
Column Descriptions:
-
N = number of sequenced bases in this gene across the individual set
-
n = number of (nonsilent) mutations in this gene across the individual set
-
npat = number of patients (individuals) with at least one nonsilent mutation
-
nsite = number of unique sites having a non-silent mutation
-
nsil = number of silent mutations in this gene across the individual set
-
n1 = number of nonsilent mutations of type: *CpG->T
-
n2 = number of nonsilent mutations of type: *Cp(A/C/T)->T
-
n3 = number of nonsilent mutations of type: A->G
-
n4 = number of nonsilent mutations of type: transver
-
n5 = number of nonsilent mutations of type: indel+null
-
n6 = number of nonsilent mutations of type: double_null
-
p_cons = p-value for enrichment of mutations at evolutionarily most-conserved sites in gene
-
p_joint = p-value for clustering + conservation
-
p = p-value (overall)
-
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 3. Get Full Table A Ranked List of Significantly Mutated Genes. Number of significant genes found: 5. Number of genes displayed: 35. Click on a gene name to display its stick figure depicting the distribution of mutations and mutation types across the chosen gene (this feature may not be available for all significant genes).
| rank | gene | description | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_cons | p_joint | p_cv | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IL32 | interleukin 32 | 61843 | 4 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.92 | 0.0002 | 1.8e-06 | 8e-09 | 0.00014 |
| 2 | CDC27 | cell division cycle 27 homolog (S. cerevisiae) | 278169 | 4 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.083 | 4.8e-06 | 0.00055 | 5.5e-08 | 0.00049 |
| 3 | NF2 | neurofibromin 2 (merlin) | 180272 | 7 | 7 | 7 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0.97 | 0.68 | 1.1e-08 | 1.5e-07 | 0.00089 |
| 4 | PPARGC1B | peroxisome proliferator-activated receptor gamma, coactivator 1 beta | 317931 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0.00046 | 0.000015 | 0.008 | 2.1e-06 | 0.0094 |
| 5 | SFRS2IP | splicing factor, arginine/serine-rich 2, interacting protein | 490729 | 5 | 5 | 2 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0.0012 | 0.00012 | 0.0011 | 2.2e-06 | 0.0094 |
| 6 | MET | met proto-oncogene (hepatocyte growth factor receptor) | 474520 | 9 | 9 | 8 | 0 | 0 | 3 | 2 | 4 | 0 | 0 | 0.0016 | 0.000066 | 0.92 | 0.00065 | 1 |
| 7 | ELF3 | E74-like factor 3 (ets domain transcription factor, epithelial-specific ) | 126731 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0.86 | 1 | 0.000097 | 0.00099 | 1 |
| 8 | PCF11 | PCF11, cleavage and polyadenylation factor subunit, homolog (S. cerevisiae) | 472280 | 8 | 7 | 7 | 3 | 0 | 1 | 4 | 3 | 0 | 0 | 0.85 | 0.00066 | 0.21 | 0.0014 | 1 |
| 9 | LGI4 | leucine-rich repeat LGI family, member 4 | 78183 | 4 | 4 | 4 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0.018 | 0.057 | 0.0029 | 0.0016 | 1 |
| 10 | RAB27B | RAB27B, member RAS oncogene family | 73617 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.0018 | 0.0018 | 1 |
| 11 | STAG2 | stromal antigen 2 | 432647 | 5 | 5 | 5 | 1 | 0 | 0 | 0 | 0 | 5 | 0 | 0.43 | 0.85 | 0.00025 | 0.002 | 1 |
| 12 | PIPOX | pipecolic acid oxidase | 133755 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.42 | 0.6 | 0.00053 | 0.0029 | 1 |
| 13 | C6orf195 | chromosome 6 open reading frame 195 | 43068 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.51 | 0.51 | 0.00081 | 0.0036 | 1 |
| 14 | ACSBG2 | acyl-CoA synthetase bubblegum family member 2 | 224342 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0.0011 | 0.42 | 0.0041 | 1 |
| 15 | BHMT | betaine-homocysteine methyltransferase | 138466 | 4 | 4 | 4 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0.1 | 0.22 | 0.0025 | 0.0046 | 1 |
| 16 | LYAR | Ly1 antibody reactive homolog (mouse) | 130092 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.12 | 0.037 | 0.021 | 0.0062 | 1 |
| 17 | LHFPL4 | lipoma HMGIC fusion partner-like 4 | 83748 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.0063 | 0.0063 | 1 |
| 18 | POMC | proopiomelanocortin (adrenocorticotropin/ beta-lipotropin/ alpha-melanocyte stimulating hormone/ beta-melanocyte stimulating hormone/ beta-endorphin) | 63547 | 3 | 3 | 3 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0.54 | 0.4 | 0.002 | 0.0064 | 1 |
| 19 | RPTN | repetin | 262185 | 3 | 3 | 3 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0.022 | 0.0034 | 0.24 | 0.0066 | 1 |
| 20 | SAV1 | salvador homolog 1 (Drosophila) | 128652 | 3 | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0.98 | 0.18 | 0.0049 | 0.0072 | 1 |
| 21 | NFE2L2 | nuclear factor (erythroid-derived 2)-like 2 | 197785 | 3 | 3 | 3 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0.013 | 0.0048 | 0.21 | 0.0079 | 1 |
| 22 | IFNA16 | interferon, alpha 16 | 63475 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0.16 | 0.048 | 0.021 | 0.0079 | 1 |
| 23 | PEBP1 | phosphatidylethanolamine binding protein 1 | 48779 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0.21 | 0.023 | 0.045 | 0.0082 | 1 |
| 24 | MCCC2 | methylcrotonoyl-Coenzyme A carboxylase 2 (beta) | 180567 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0.035 | 0.018 | 0.068 | 0.0096 | 1 |
| 25 | POU4F2 | POU class 4 homeobox 2 | 120647 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.99 | 0.18 | 0.0074 | 0.01 | 1 |
| 26 | PARD6B | par-6 partitioning defective 6 homolog beta (C. elegans) | 117771 | 4 | 4 | 4 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0.74 | 0.98 | 0.0015 | 0.011 | 1 |
| 27 | VPS37D | vacuolar protein sorting 37 homolog D (S. cerevisiae) | 30362 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.011 | 0.011 | 1 |
| 28 | STARD3 | StAR-related lipid transfer (START) domain containing 3 | 152873 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.059 | 0.088 | 0.019 | 0.012 | 1 |
| 29 | WFDC10A | WAP four-disulfide core domain 10A | 27441 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.015 | 0.015 | 1 |
| 30 | LRFN4 | leucine rich repeat and fibronectin type III domain containing 4 | 130619 | 3 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0.69 | 1 | 0.0022 | 0.016 | 1 |
| 31 | TMEM37 | transmembrane protein 37 | 61716 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | NaN | NaN | 0.017 | 0.017 | 1 |
| 32 | SEPT7 | septin 7 | 96217 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.88 | 1 | 0.003 | 0.02 | 1 |
| 33 | C10orf95 | chromosome 10 open reading frame 95 | 29115 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.021 | 0.021 | 1 |
| 34 | C15orf52 | chromosome 15 open reading frame 52 | 156736 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | NaN | NaN | 0.021 | 0.021 | 1 |
| 35 | KDELR3 | KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 3 | 80347 | 2 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.14 | 0.18 | 0.018 | 0.022 | 1 |
Figure S1. This figure depicts the distribution of mutations and mutation types across the IL32 significant gene.
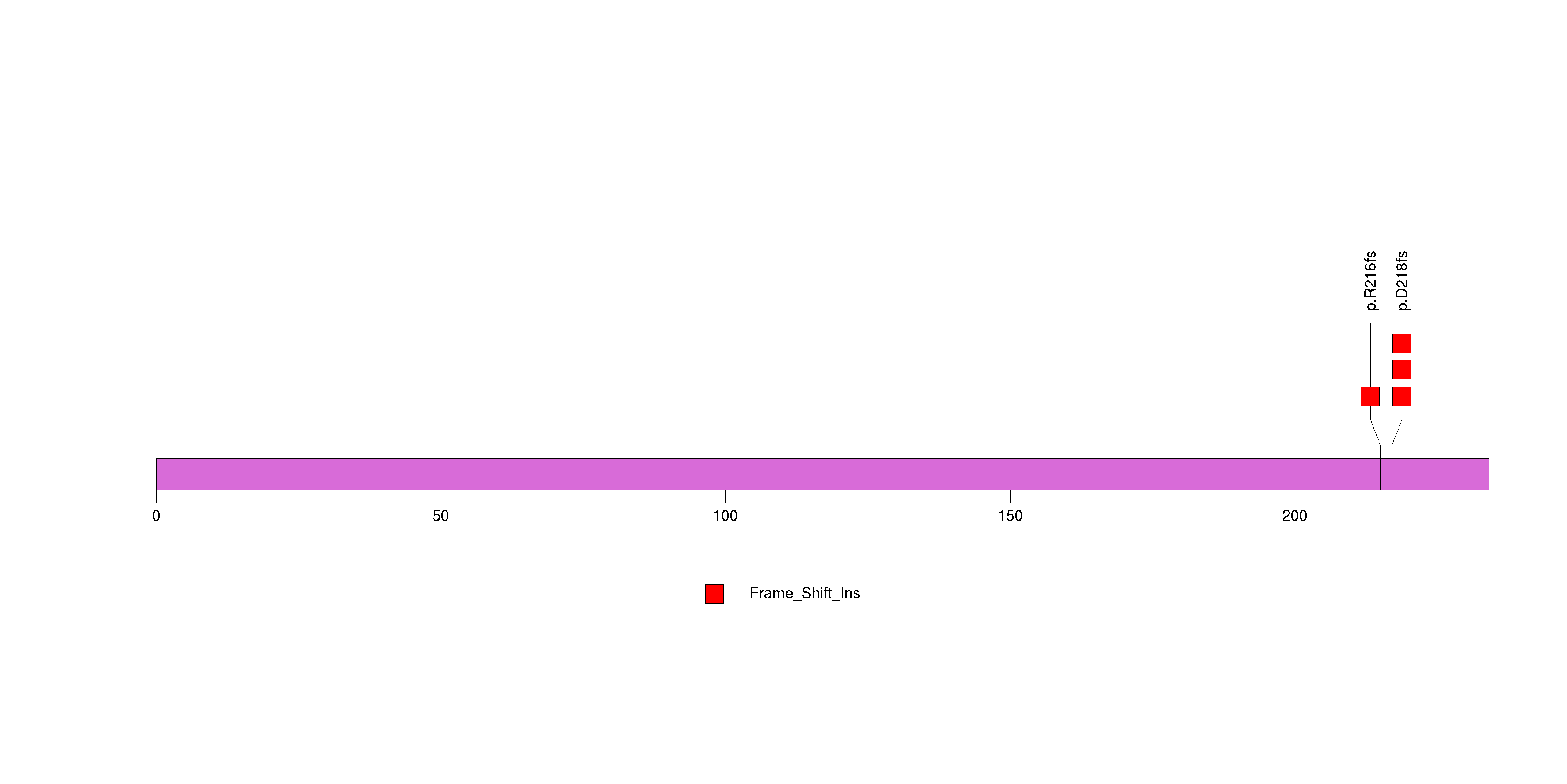
Figure S2. This figure depicts the distribution of mutations and mutation types across the CDC27 significant gene.
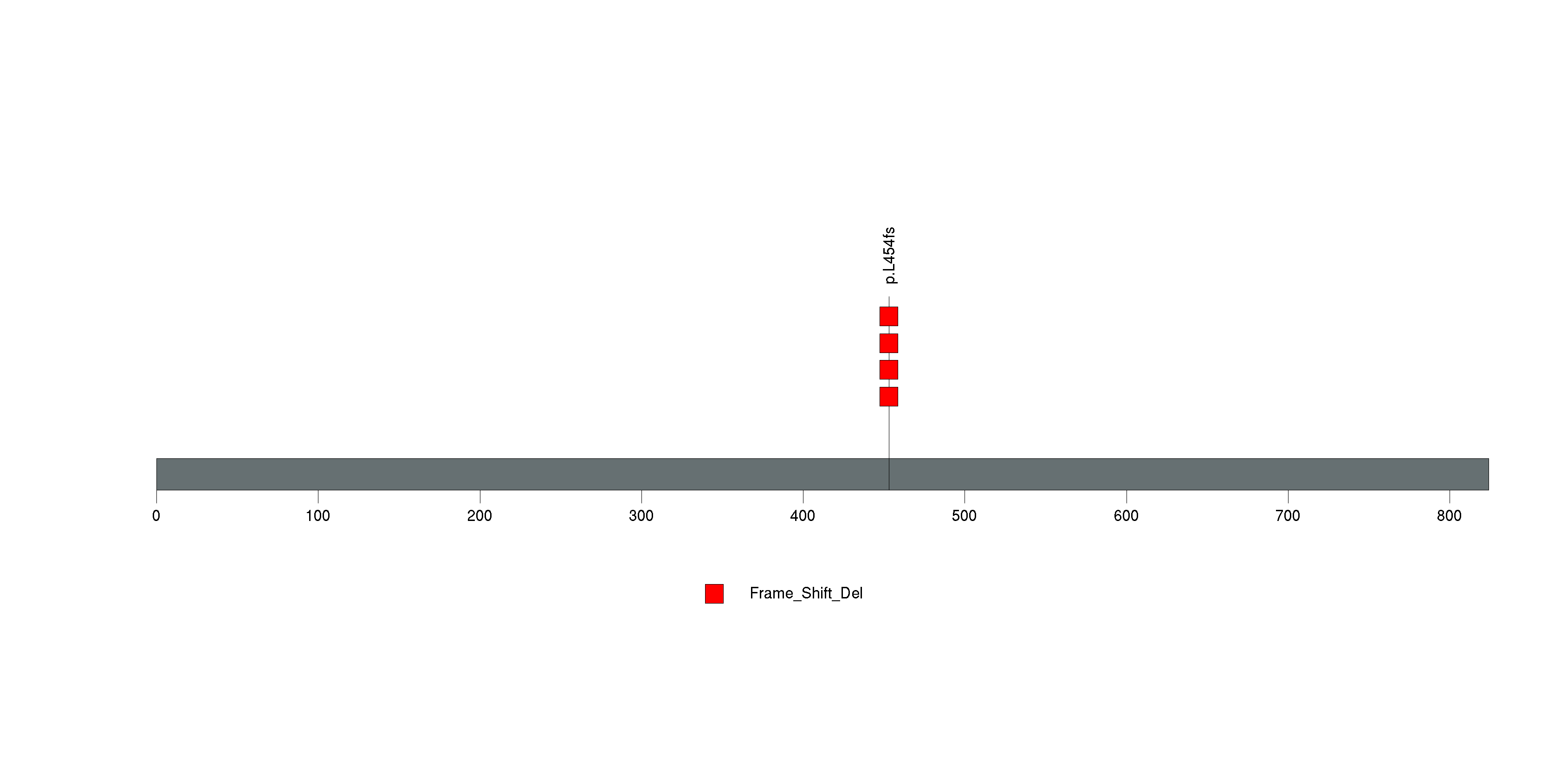
Figure S3. This figure depicts the distribution of mutations and mutation types across the NF2 significant gene.
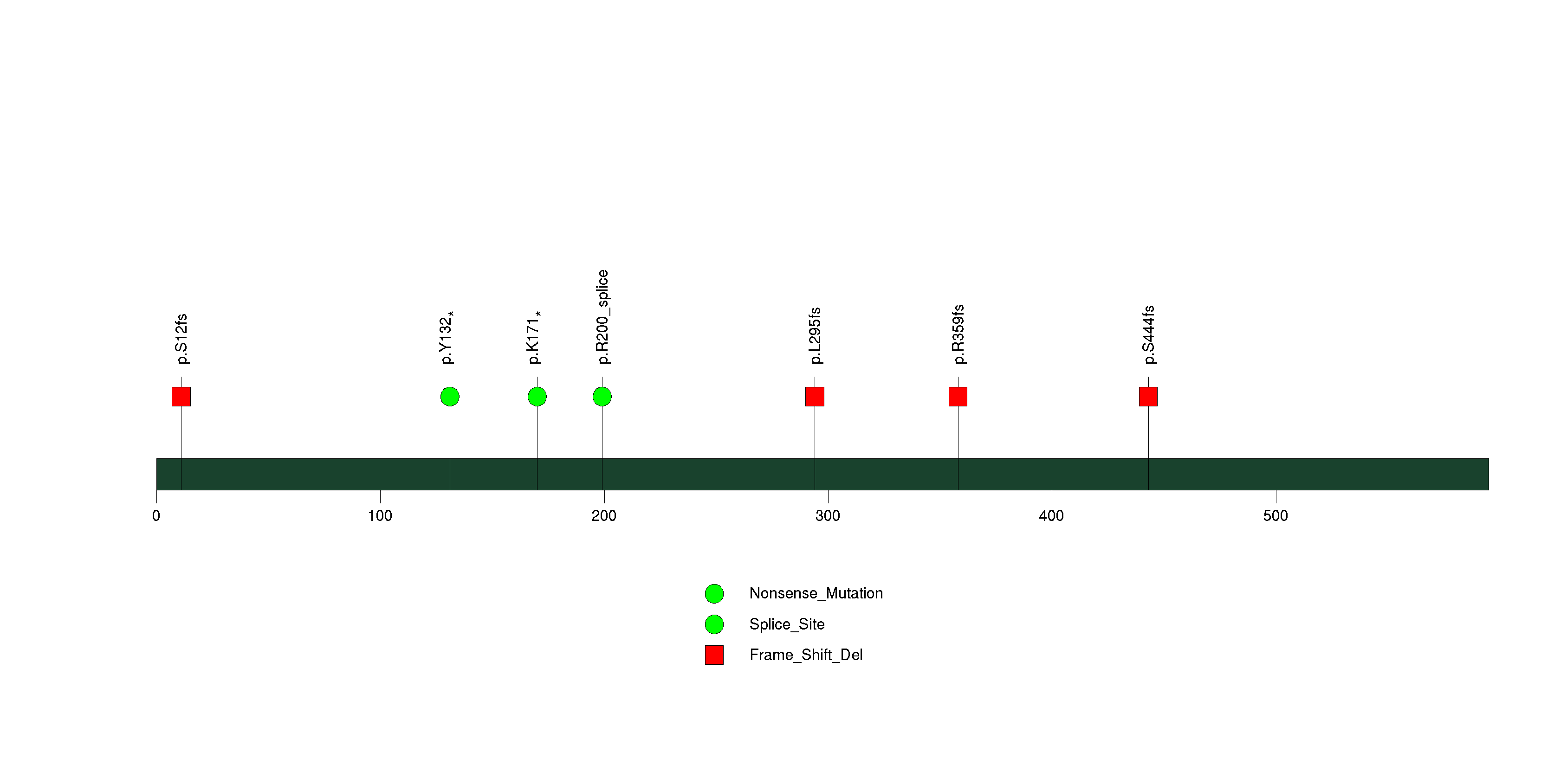
Figure S4. This figure depicts the distribution of mutations and mutation types across the PPARGC1B significant gene.
In this analysis, COSMIC is used as a filter to increase power by restricting the territory of each gene. Cosmic version: v48.
Table 4. Get Full Table Significantly mutated genes (COSMIC territory only). To access the database please go to: COSMIC. Number of significant genes found: 11. Number of genes displayed: 10
| rank | gene | description | n | cos | n_cos | N_cos | cos_ev | p | q |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MET | met proto-oncogene (hepatocyte growth factor receptor) | 9 | 34 | 4 | 3774 | 12 | 1e-10 | 4.7e-07 |
| 2 | FGFR3 | fibroblast growth factor receptor 3 (achondroplasia, thanatophoric dwarfism) | 5 | 62 | 3 | 6882 | 1469 | 3.6e-07 | 0.00081 |
| 3 | NF2 | neurofibromin 2 (merlin) | 7 | 550 | 4 | 61050 | 29 | 6.6e-06 | 0.0099 |
| 4 | SMARCA4 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 | 5 | 30 | 2 | 3330 | 3 | 0.000019 | 0.022 |
| 5 | KRAS | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | 2 | 52 | 2 | 5772 | 29208 | 0.000058 | 0.053 |
| 6 | BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 4 | 89 | 2 | 9879 | 14380 | 0.00017 | 0.086 |
| 7 | CDCA8 | cell division cycle associated 8 | 1 | 1 | 1 | 111 | 1 | 0.00021 | 0.086 |
| 8 | FLCN | folliculin | 1 | 1 | 1 | 111 | 1 | 0.00021 | 0.086 |
| 9 | G6PC | glucose-6-phosphatase, catalytic subunit | 1 | 1 | 1 | 111 | 1 | 0.00021 | 0.086 |
| 10 | PLXDC2 | plexin domain containing 2 | 1 | 1 | 1 | 111 | 1 | 0.00021 | 0.086 |
Note:
n - number of (nonsilent) mutations in this gene across the individual set.
cos = number of unique mutated sites in this gene in COSMIC
n_cos = overlap between n and cos.
N_cos = number of individuals times cos.
cos_ev = total evidence: number of reports in COSMIC for mutations seen in this gene.
p = p-value for seeing the observed amount of overlap in this gene)
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 5. Get Full Table A Ranked List of Significantly Mutated Genesets. (Source: MSigDB GSEA Cannonical Pathway Set).Number of significant genesets found: 0. Number of genesets displayed: 10
| rank | geneset | description | genes | N_genes | mut_tally | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_ns_s | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MTORPATHWAY | Mammalian target of rapamycin (mTOR) senses mitogenic factors and nutrients, including ATP, and induces cell proliferation. | AKT1, EIF3S10, EIF4A1, EIF4A2, EIF4B, EIF4E, EIF4EBP1, EIF4G1, EIF4G2, EIF4G3, FKBP1A, FRAP1, MKNK1, PDK2, PDPK1, PIK3CA, PIK3R1, PPP2CA, PTEN, RPS6, RPS6KB1, TSC1, TSC2 | 21 | EIF4A1(1), EIF4B(2), EIF4G1(2), EIF4G3(4), PIK3CA(2), PIK3R1(1), PTEN(3), TSC1(2), TSC2(4) | 4612682 | 21 | 19 | 21 | 2 | 2 | 6 | 3 | 3 | 7 | 0 | 0.047 | 0.0011 | 0.66 |
| 2 | ALANINE_AND_ASPARTATE_METABOLISM | AARS, ABAT, ADSL, ADSS, AGXT, AGXT2, ASL, ASNS, ASPA, ASS, CAD, CRAT, DARS, DDO, GAD1, GAD2, GOT1, GOT2, GPT, GPT2, NARS, PC | 21 | AARS(1), ADSL(1), AGXT(1), AGXT2(1), ASNS(1), CAD(3), CRAT(1), DARS(3), DDO(1), GPT(2), PC(4) | 4337938 | 19 | 17 | 19 | 1 | 0 | 3 | 1 | 8 | 7 | 0 | 0.078 | 0.0034 | 1 | |
| 3 | CBLPATHWAY | Activated EGF receptors undergo endocytosis into clathrin-coated vesicles, where they are recycled to the membrane or ubiquitinated by Cbl. | CBL, CSF1R, EGF, EGFR, GRB2, MET, PDGFRA, PRKCA, PRKCB1, SH3GLB1, SH3GLB2, SH3KBP1, SRC | 12 | CSF1R(1), EGF(1), MET(9), PDGFRA(2) | 3225375 | 13 | 12 | 12 | 1 | 1 | 3 | 2 | 6 | 1 | 0 | 0.11 | 0.0074 | 1 |
| 4 | NEUTROPHILPATHWAY | Neutrophils are phagocytotic leukocytes that destroy foreign cells with reactive oxygen species or enzymatic digestion and express CD11 and CD18. | CD44, ICAM1, ITGAL, ITGAM, ITGB2, PECAM1, SELE, SELL | 8 | ITGAL(3), ITGAM(2), ITGB2(1), SELE(1), SELL(1) | 1726164 | 8 | 8 | 8 | 0 | 1 | 2 | 0 | 3 | 2 | 0 | 0.13 | 0.01 | 1 |
| 5 | KREBPATHWAY | The Krebs (citric acid) cycle takes place in mitochondria, where it extracts energy in the form of electron carriers NADH and FADH2, which drive the electron transport chain. | ACO2, CS, FH, IDH2, MDH1, OGDH, SDHA, SUCLA2 | 8 | FH(1), IDH2(1), MDH1(1), OGDH(2), SDHA(2) | 1558671 | 7 | 7 | 7 | 0 | 0 | 3 | 0 | 1 | 3 | 0 | 0.072 | 0.01 | 1 |
| 6 | LYMPHOCYTEPATHWAY | B and T cell lymphocytes interact with other cells via transmembrane adhesion proteins such as CD44, which interacts with endothelial cells. | CD44, ICAM1, ITGA4, ITGAL, ITGB1, ITGB2, PECAM1, SELE, SELL | 9 | ITGA4(1), ITGAL(3), ITGB1(1), ITGB2(1), SELE(1), SELL(1) | 1999098 | 8 | 8 | 8 | 0 | 1 | 2 | 1 | 1 | 3 | 0 | 0.15 | 0.011 | 1 |
| 7 | MONOCYTEPATHWAY | Monocytes are a class of immune phagocytes that can develop into macrophages and express LFA-1, CD44, and other surface signaling proteins. | CD44, ICAM1, ITGA4, ITGAL, ITGAM, ITGB1, ITGB2, PECAM1, SELE, SELL, SELP | 11 | ITGA4(1), ITGAL(3), ITGAM(2), ITGB1(1), ITGB2(1), SELE(1), SELL(1) | 2640660 | 10 | 10 | 10 | 0 | 1 | 2 | 1 | 3 | 3 | 0 | 0.11 | 0.014 | 1 |
| 8 | DNAFRAGMENTPATHWAY | DNA fragmentation during apoptosis is effected by DFF, a caspase-activated DNAse, and by endonuclease G. | CASP3, CASP7, DFFA, DFFB, ENDOG, GZMB, HMGB1, HMGB2, TOP2A, TOP2B | 9 | HMGB1(3), HMGB2(2), TOP2B(3) | 1504641 | 8 | 7 | 8 | 0 | 1 | 1 | 1 | 3 | 2 | 0 | 0.21 | 0.016 | 1 |
| 9 | HSA00830_RETINOL_METABOLISM | Genes involved in retinol metabolism | ALDH1A1, ALDH1A2, BCMO1, RDH5 | 4 | BCMO1(2), RDH5(2) | 643601 | 4 | 4 | 4 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0.72 | 0.017 | 1 |
| 10 | RABPATHWAY | Rab family GTPases regulate vesicle transport, endocytosis and exocytosis, and vesicle docking via interactions with the rabphilins. | ACTA1, MEL, RAB11A, RAB1A, RAB2, RAB27A, RAB3A, RAB4A, RAB5A, RAB6A, RAB7, RAB9A | 9 | ACTA1(1), RAB11A(1), RAB3A(1), RAB6A(1) | 705580 | 4 | 4 | 4 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0.81 | 0.021 | 1 |
In brief, we tabulate the number of mutations and the number of covered bases for each gene. The counts are broken down by mutation context category: four context categories that are discovered by MutSig, and one for indel and 'null' mutations, which include indels, nonsense mutations, splice-site mutations, and non-stop (read-through) mutations. For each gene, we calculate the probability of seeing the observed constellation of mutations, i.e. the product P1 x P2 x ... x Pm, or a more extreme one, given the background mutation rates calculated across the dataset. [1]
This is an experimental feature. The full results of the analysis summarized in this report can be downloaded from the TCGA Data Coordination Center.