This report serves to describe the mutational landscape and properties of a given individual set, as well as rank genes and genesets according to mutational significance. MutSig v2.0 was used to generate the results found in this report.
-
Working with individual set: LUSC-TP
-
Number of patients in set: 178
The input for this pipeline is a set of individuals with the following files associated for each:
-
An annotated .maf file describing the mutations called for the respective individual, and their properties.
-
A .wig file that contains information about the coverage of the sample.
-
MAF used for this analysis:LUSC-TP.final_analysis_set.maf
-
Blacklist used for this analysis: pancan_mutation_blacklist.v14.hg19.txt
-
Significantly mutated genes (q ≤ 0.1): 114
-
Mutations seen in COSMIC: 350
-
Significantly mutated genes in COSMIC territory: 11
-
Significantly mutated genesets: 19
-
Significantly mutated genesets: (excluding sig. mutated genes):0
-
Read 178 MAFs of type "maf1"
-
Total number of mutations in input MAFs: 64361
-
After removing 2 mutations outside chr1-24: 64359
-
After removing 591 blacklisted mutations: 63768
-
After removing 1436 noncoding mutations: 62332
-
Number of mutations before filtering: 62332
-
After removing 3143 mutations outside gene set: 59189
-
After removing 42 mutations outside category set: 59147
-
After removing 2 "impossible" mutations in
-
gene-patient-category bins of zero coverage: 55960
Table 1. Get Full Table Table representing breakdown of mutations by type.
| type | count |
|---|---|
| De_novo_Start_InFrame | 5 |
| De_novo_Start_OutOfFrame | 12 |
| Frame_Shift_Del | 474 |
| Frame_Shift_Ins | 108 |
| In_Frame_Del | 42 |
| In_Frame_Ins | 3 |
| Missense_Mutation | 38628 |
| Nonsense_Mutation | 3357 |
| Nonstop_Mutation | 52 |
| Silent | 14139 |
| Splice_Site | 2251 |
| Start_Codon_Del | 1 |
| Start_Codon_SNP | 74 |
| Stop_Codon_Ins | 1 |
| Total | 59147 |
Table 2. Get Full Table A breakdown of mutation rates per category discovered for this individual set.
| category | n | N | rate | rate_per_mb | relative_rate | exp_ns_s_ratio |
|---|---|---|---|---|---|---|
| Tp*C->mut | 12013 | 692977947 | 0.000017 | 17 | 2 | 3.3 |
| (A/C/G)p*C->(A/T) | 15606 | 1964339776 | 7.9e-06 | 7.9 | 0.92 | 2.6 |
| (A/C/G)p*C->G | 3037 | 1964339776 | 1.5e-06 | 1.5 | 0.18 | 4.9 |
| A->mut | 8046 | 2561387954 | 3.1e-06 | 3.1 | 0.36 | 3.9 |
| indel+null | 6265 | 5218705677 | 1.2e-06 | 1.2 | 0.14 | NaN |
| double_null | 40 | 5218705677 | 7.7e-09 | 0.0077 | 0.00089 | NaN |
| Total | 45007 | 5218705677 | 8.6e-06 | 8.6 | 1 | 3.5 |
The x axis represents the samples. The y axis represents the exons, one row per exon, and they are sorted by average coverage across samples. For exons with exactly the same average coverage, they are sorted next by the %GC of the exon. (The secondary sort is especially useful for the zero-coverage exons at the bottom). If the figure is unpopulated, then full coverage is assumed (e.g. MutSig CV doesn't use WIGs and assumes full coverage).
Figure 1.

Figure 2. Patients counts and rates file used to generate this plot: LUSC-TP.patients.counts_and_rates.txt

The mutation spectrum is depicted in the lego plots below in which the 96 possible mutation types are subdivided into six large blocks, color-coded to reflect the base substitution type. Each large block is further subdivided into the 16 possible pairs of 5' and 3' neighbors, as listed in the 4x4 trinucleotide context legend. The height of each block corresponds to the mutation frequency for that kind of mutation (counts of mutations normalized by the base coverage in a given bin). The shape of the spectrum is a signature for dominant mutational mechanisms in different tumor types.
Figure 3. Get High-res Image SNV Mutation rate lego plot for entire set. Each bin is normalized by base coverage for that bin. Colors represent the six SNV types on the upper right. The three-base context for each mutation is labeled in the 4x4 legend on the lower right. The fractional breakdown of SNV counts is shown in the pie chart on the upper left. If this figure is blank, not enough information was provided in the MAF to generate it.

Figure 4. Get High-res Image SNV Mutation rate lego plots for 4 slices of mutation allele fraction (0<=AF<0.1, 0.1<=AF<0.25, 0.25<=AF<0.5, & 0.5<=AF) . The color code and three-base context legends are the same as the previous figure. If this figure is blank, not enough information was provided in the MAF to generate it.

Column Descriptions:
-
N = number of sequenced bases in this gene across the individual set
-
n = number of (nonsilent) mutations in this gene across the individual set
-
npat = number of patients (individuals) with at least one nonsilent mutation
-
nsite = number of unique sites having a non-silent mutation
-
nsil = number of silent mutations in this gene across the individual set
-
n1 = number of nonsilent mutations of type: Tp*C->mut
-
n2 = number of nonsilent mutations of type: (A/C/G)p*C->(A/T)
-
n3 = number of nonsilent mutations of type: (A/C/G)p*C->G
-
n4 = number of nonsilent mutations of type: A->mut
-
n5 = number of nonsilent mutations of type: indel+null
-
n6 = number of nonsilent mutations of type: double_null
-
p_classic = p-value for the observed amount of nonsilent mutations being elevated in this gene
-
p_ns_s = p-value for the observed nonsilent/silent ratio being elevated in this gene
-
p_cons = p-value for enrichment of mutations at evolutionarily most-conserved sites in gene
-
p_joint = p-value for clustering + conservation
-
p = p-value (overall)
-
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 3. Get Full Table A Ranked List of Significantly Mutated Genes. Number of significant genes found: 114. Number of genes displayed: 35. Click on a gene name to display its stick figure depicting the distribution of mutations and mutation types across the chosen gene (this feature may not be available for all significant genes).
| rank | gene | description | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_classic | p_ns_s | p_clust | p_cons | p_joint | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CDKN2A | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | 134387 | 26 | 26 | 23 | 1 | 5 | 3 | 1 | 2 | 15 | 0 | 3.6e-15 | 0.016 | 0.0076 | 6.8e-06 | 3.8e-06 | 0.000 | 0.000 |
| 2 | TP53 | tumor protein p53 | 218677 | 149 | 145 | 97 | 1 | 8 | 40 | 16 | 32 | 52 | 1 | 1.3e-15 | 1e-14 | 0 | 0 | 0 | <1.00e-15 | <4.50e-12 |
| 3 | NFE2L2 | nuclear factor (erythroid-derived 2)-like 2 | 318204 | 28 | 27 | 15 | 0 | 19 | 4 | 0 | 4 | 1 | 0 | 1.4e-15 | 0.007 | 0 | 0 | 0 | <1.00e-15 | <4.50e-12 |
| 4 | PIK3CA | phosphoinositide-3-kinase, catalytic, alpha polypeptide | 583734 | 29 | 27 | 16 | 1 | 20 | 3 | 0 | 5 | 1 | 0 | 4.1e-13 | 0.021 | 0.000091 | 0.0018 | 0 | <1.00e-15 | <4.50e-12 |
| 5 | TPTE | transmembrane phosphatase with tensin homology | 308873 | 30 | 24 | 29 | 2 | 9 | 3 | 2 | 8 | 8 | 0 | 7.7e-15 | 0.034 | 0.61 | 0.42 | 0.54 | 1.40e-13 | 5.03e-10 |
| 6 | KEAP1 | kelch-like ECH-associated protein 1 | 312991 | 24 | 22 | 21 | 0 | 9 | 9 | 2 | 2 | 2 | 0 | 1.2e-13 | 0.00021 | 0.036 | 0.18 | 0.047 | 1.86e-13 | 5.58e-10 |
| 7 | SI | sucrase-isomaltase (alpha-glucosidase) | 995567 | 46 | 36 | 46 | 2 | 10 | 20 | 2 | 8 | 6 | 0 | 1.6e-12 | 0.0066 | 0.78 | 0.85 | 1 | 4.52e-11 | 1.16e-07 |
| 8 | PTEN | phosphatase and tensin homolog (mutated in multiple advanced cancers 1) | 214719 | 16 | 14 | 15 | 0 | 4 | 1 | 0 | 3 | 8 | 0 | 7.4e-11 | 0.1 | 0.044 | 0.46 | 0.075 | 1.50e-10 | 3.37e-07 |
| 9 | TRIM58 | tripartite motif-containing 58 | 195196 | 16 | 15 | 16 | 1 | 2 | 8 | 1 | 1 | 4 | 0 | 4.4e-10 | 0.04 | 0.06 | 0.67 | 0.16 | 1.69e-09 | 3.38e-06 |
| 10 | OR5L2 | olfactory receptor, family 5, subfamily L, member 2 | 167267 | 15 | 13 | 15 | 1 | 0 | 6 | 3 | 4 | 2 | 0 | 4.4e-10 | 0.068 | 0.39 | 0.38 | 0.52 | 5.23e-09 | 9.42e-06 |
| 11 | REG1B | regenerating islet-derived 1 beta (pancreatic stone protein, pancreatic thread protein) | 92608 | 11 | 11 | 9 | 1 | 5 | 2 | 0 | 1 | 3 | 0 | 8e-10 | 0.2 | 0.34 | 0.2 | 0.37 | 6.72e-09 | 1.10e-05 |
| 12 | LRRC4C | leucine rich repeat containing 4C | 342685 | 19 | 17 | 19 | 1 | 5 | 8 | 1 | 4 | 1 | 0 | 7.1e-09 | 0.021 | 0.073 | 0.19 | 0.063 | 1.00e-08 | 1.51e-05 |
| 13 | ELTD1 | EGF, latrophilin and seven transmembrane domain containing 1 | 365327 | 18 | 18 | 18 | 1 | 4 | 2 | 1 | 4 | 7 | 0 | 1.8e-09 | 0.08 | 0.5 | 0.82 | 0.65 | 2.49e-08 | 3.45e-05 |
| 14 | ZBBX | zinc finger, B-box domain containing | 437517 | 17 | 17 | 17 | 1 | 4 | 3 | 1 | 5 | 4 | 0 | 3.3e-08 | 0.13 | 0.023 | 0.77 | 0.052 | 3.66e-08 | 4.13e-05 |
| 15 | PDYN | prodynorphin | 137367 | 10 | 10 | 10 | 1 | 2 | 5 | 2 | 1 | 0 | 0 | 2.8e-07 | 0.14 | 0.0058 | 0.18 | 0.0062 | 3.66e-08 | 4.13e-05 |
| 16 | OR2G6 | olfactory receptor, family 2, subfamily G, member 6 | 169883 | 17 | 16 | 17 | 3 | 2 | 6 | 1 | 7 | 1 | 0 | 2.2e-08 | 0.16 | 0.11 | 0.096 | 0.08 | 3.67e-08 | 4.13e-05 |
| 17 | DPPA4 | developmental pluripotency associated 4 | 167827 | 12 | 12 | 12 | 0 | 4 | 3 | 1 | 0 | 4 | 0 | 8.7e-09 | 0.057 | 0.31 | 0.48 | 0.43 | 7.61e-08 | 8.06e-05 |
| 18 | OR4M2 | olfactory receptor, family 4, subfamily M, member 2 | 168054 | 14 | 14 | 14 | 2 | 2 | 6 | 0 | 5 | 1 | 0 | 4.1e-09 | 0.2 | 0.92 | 0.84 | 1 | 8.34e-08 | 8.35e-05 |
| 19 | ASB5 | ankyrin repeat and SOCS box-containing 5 | 180540 | 9 | 9 | 9 | 0 | 3 | 1 | 2 | 2 | 1 | 0 | 5.9e-06 | 0.14 | 0.012 | 0.0053 | 0.0014 | 1.59e-07 | 0.000151 |
| 20 | CYP11B1 | cytochrome P450, family 11, subfamily B, polypeptide 1 | 272922 | 15 | 15 | 15 | 0 | 0 | 11 | 1 | 3 | 0 | 0 | 6.7e-08 | 0.0059 | 0.094 | 0.92 | 0.18 | 2.33e-07 | 0.000210 |
| 21 | CRB1 | crumbs homolog 1 (Drosophila) | 759065 | 27 | 23 | 27 | 2 | 8 | 6 | 1 | 9 | 3 | 0 | 7.4e-08 | 0.043 | 0.14 | 0.82 | 0.22 | 3.05e-07 | 0.000261 |
| 22 | OR6F1 | olfactory receptor, family 6, subfamily F, member 1 | 165718 | 13 | 13 | 13 | 2 | 2 | 6 | 1 | 4 | 0 | 0 | 1.7e-07 | 0.19 | 0.33 | 0.14 | 0.22 | 6.85e-07 | 0.000560 |
| 23 | CPS1 | carbamoyl-phosphate synthetase 1, mitochondrial | 828677 | 28 | 24 | 27 | 2 | 4 | 15 | 1 | 6 | 2 | 0 | 1.8e-06 | 0.02 | 0.03 | 0.74 | 0.054 | 1.63e-06 | 0.00127 |
| 24 | OR2T33 | olfactory receptor, family 2, subfamily T, member 33 | 171450 | 15 | 14 | 15 | 3 | 2 | 7 | 1 | 4 | 1 | 0 | 1.3e-07 | 0.22 | 0.99 | 0.39 | 0.81 | 1.74e-06 | 0.00131 |
| 25 | ESRRG | estrogen-related receptor gamma | 250009 | 12 | 12 | 12 | 1 | 2 | 5 | 1 | 2 | 2 | 0 | 7.8e-07 | 0.13 | 0.09 | 0.57 | 0.15 | 1.97e-06 | 0.00142 |
| 26 | REG3A | regenerating islet-derived 3 alpha | 97465 | 14 | 11 | 14 | 2 | 1 | 8 | 3 | 1 | 1 | 0 | 9.4e-07 | 0.16 | 0.085 | 0.73 | 0.14 | 2.30e-06 | 0.00155 |
| 27 | PNLIPRP3 | pancreatic lipase-related protein 3 | 257837 | 12 | 12 | 12 | 1 | 1 | 6 | 2 | 1 | 2 | 0 | 3.6e-07 | 0.33 | 0.44 | 0.21 | 0.38 | 2.32e-06 | 0.00155 |
| 28 | SPHKAP | SPHK1 interactor, AKAP domain containing | 911279 | 35 | 27 | 35 | 3 | 4 | 17 | 5 | 8 | 1 | 0 | 1.7e-07 | 0.015 | 0.85 | 0.82 | 1 | 2.80e-06 | 0.00180 |
| 29 | COL22A1 | collagen, type XXII, alpha 1 | 808953 | 35 | 30 | 35 | 4 | 4 | 15 | 2 | 4 | 9 | 1 | 2.5e-07 | 0.059 | 0.98 | 0.29 | 0.75 | 3.03e-06 | 0.00188 |
| 30 | MAGEB2 | melanoma antigen family B, 2 | 133192 | 9 | 9 | 9 | 1 | 2 | 4 | 2 | 1 | 0 | 0 | 3e-07 | 0.26 | 0.63 | 0.56 | 0.78 | 3.86e-06 | 0.00232 |
| 31 | SLC13A1 | solute carrier family 13 (sodium/sulfate symporters), member 1 | 326098 | 12 | 12 | 12 | 1 | 4 | 2 | 1 | 4 | 1 | 0 | 2.1e-06 | 0.15 | 0.072 | 0.52 | 0.15 | 5.09e-06 | 0.00296 |
| 32 | OR51B2 | olfactory receptor, family 51, subfamily B, member 2 | 166648 | 11 | 10 | 11 | 0 | 2 | 4 | 1 | 3 | 1 | 0 | 7e-07 | 0.059 | 0.48 | 0.47 | 0.6 | 6.62e-06 | 0.00372 |
| 33 | TGIF2LX | TGFB-induced factor homeobox 2-like, X-linked | 129265 | 11 | 9 | 11 | 1 | 2 | 2 | 0 | 5 | 2 | 0 | 9.2e-07 | 0.25 | 0.42 | 0.52 | 0.59 | 8.34e-06 | 0.00455 |
| 34 | USP29 | ubiquitin specific peptidase 29 | 493525 | 17 | 16 | 17 | 1 | 3 | 6 | 2 | 3 | 3 | 0 | 7.6e-07 | 0.091 | 0.84 | 0.45 | 0.86 | 9.92e-06 | 0.00525 |
| 35 | ASCL4 | achaete-scute complex homolog 4 (Drosophila) | 49103 | 6 | 6 | 6 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 2.3e-06 | 0.12 | 0.34 | 0.22 | 0.34 | 1.20e-05 | 0.00599 |
Figure S1. This figure depicts the distribution of mutations and mutation types across the CDKN2A significant gene.

Figure S2. This figure depicts the distribution of mutations and mutation types across the TP53 significant gene.

Figure S3. This figure depicts the distribution of mutations and mutation types across the NFE2L2 significant gene.

Figure S4. This figure depicts the distribution of mutations and mutation types across the PIK3CA significant gene.

Figure S5. This figure depicts the distribution of mutations and mutation types across the TPTE significant gene.
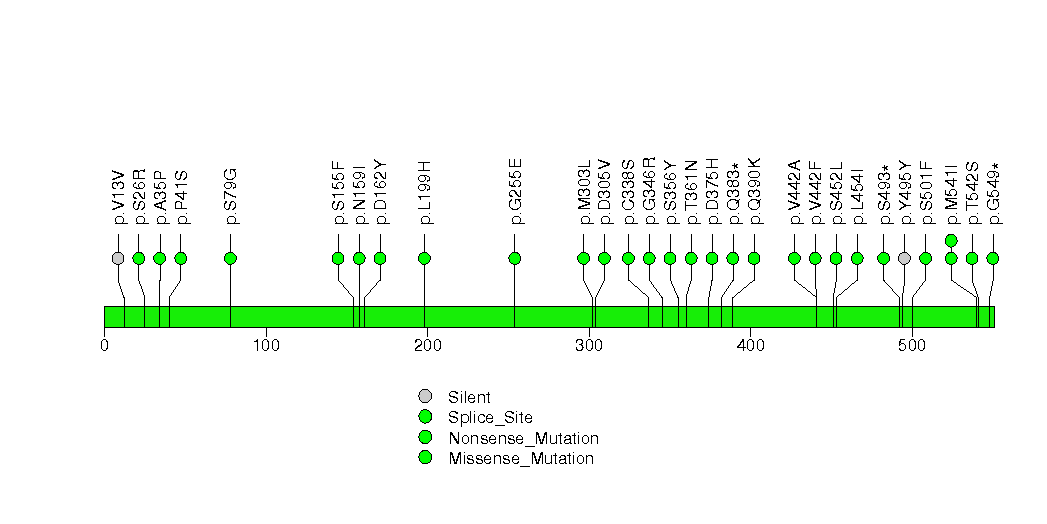
Figure S6. This figure depicts the distribution of mutations and mutation types across the KEAP1 significant gene.
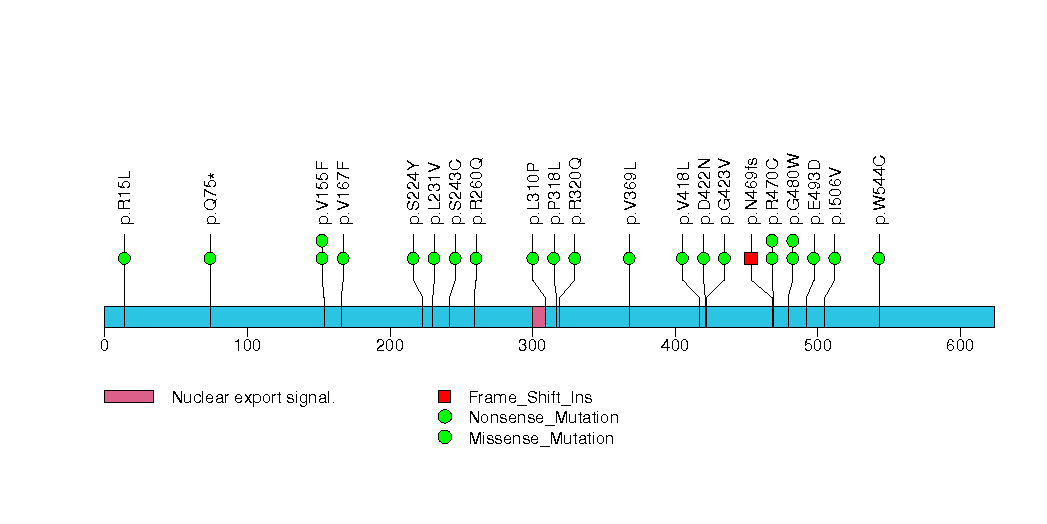
Figure S7. This figure depicts the distribution of mutations and mutation types across the SI significant gene.
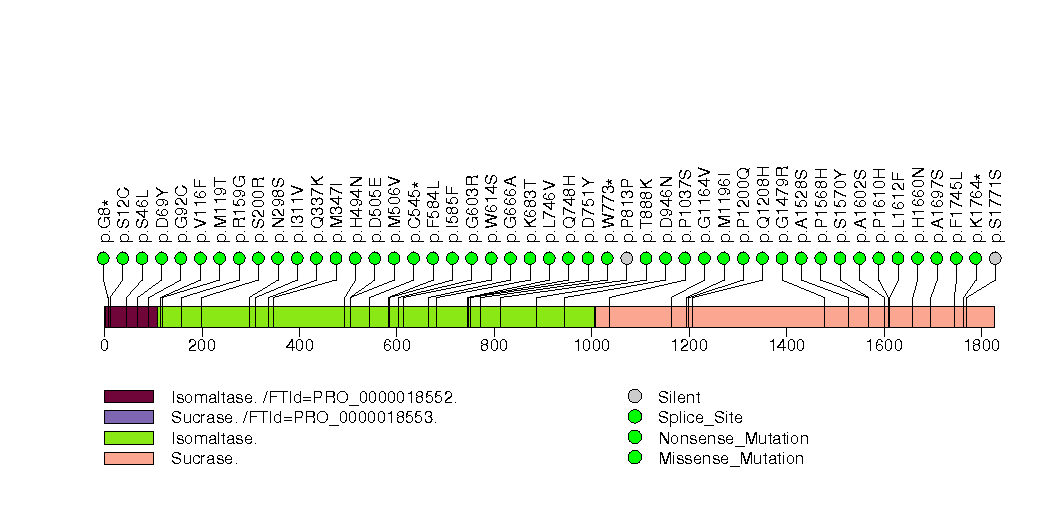
Figure S8. This figure depicts the distribution of mutations and mutation types across the PTEN significant gene.

Figure S9. This figure depicts the distribution of mutations and mutation types across the TRIM58 significant gene.

Figure S10. This figure depicts the distribution of mutations and mutation types across the OR5L2 significant gene.

Figure S11. This figure depicts the distribution of mutations and mutation types across the REG1B significant gene.
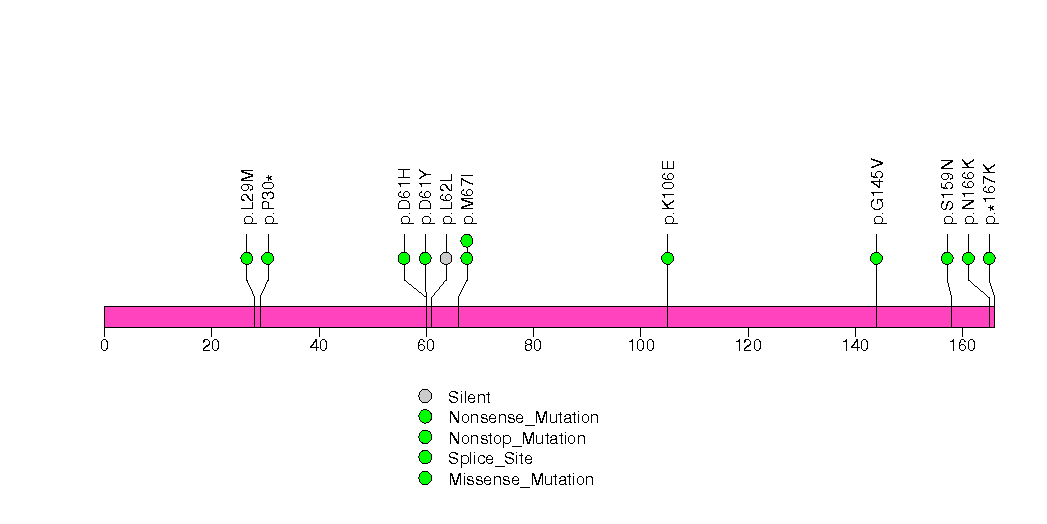
Figure S12. This figure depicts the distribution of mutations and mutation types across the LRRC4C significant gene.
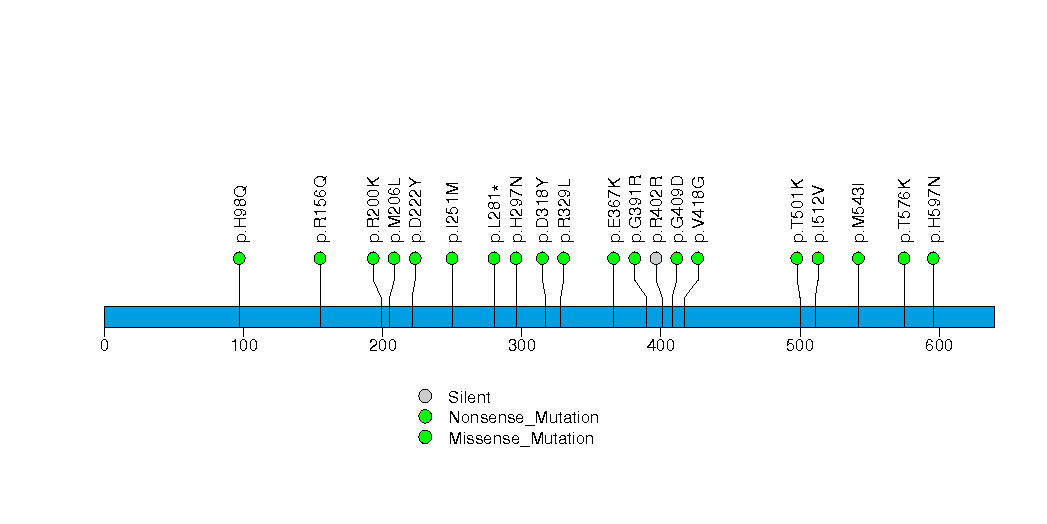
Figure S13. This figure depicts the distribution of mutations and mutation types across the ELTD1 significant gene.
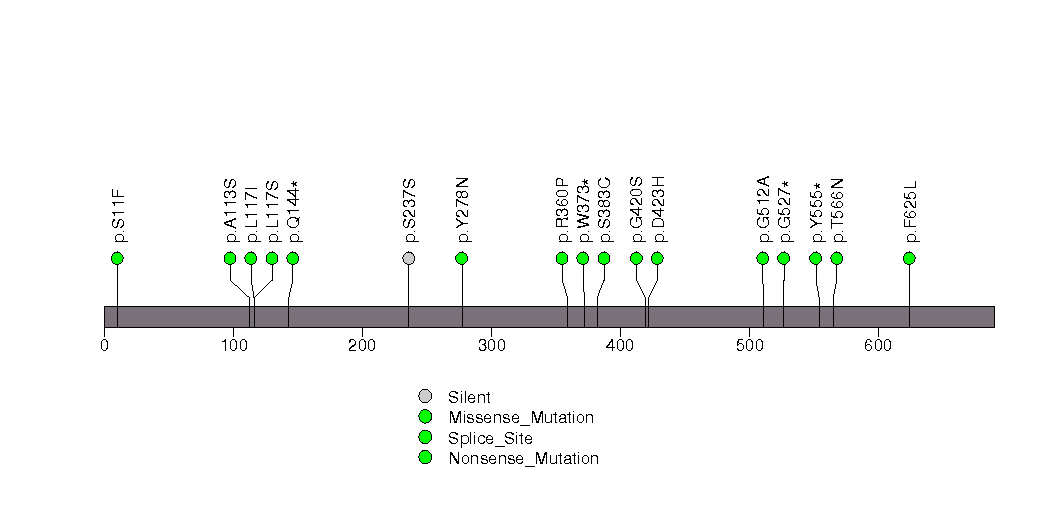
Figure S14. This figure depicts the distribution of mutations and mutation types across the ZBBX significant gene.

Figure S15. This figure depicts the distribution of mutations and mutation types across the OR2G6 significant gene.

Figure S16. This figure depicts the distribution of mutations and mutation types across the DPPA4 significant gene.

Figure S17. This figure depicts the distribution of mutations and mutation types across the OR4M2 significant gene.
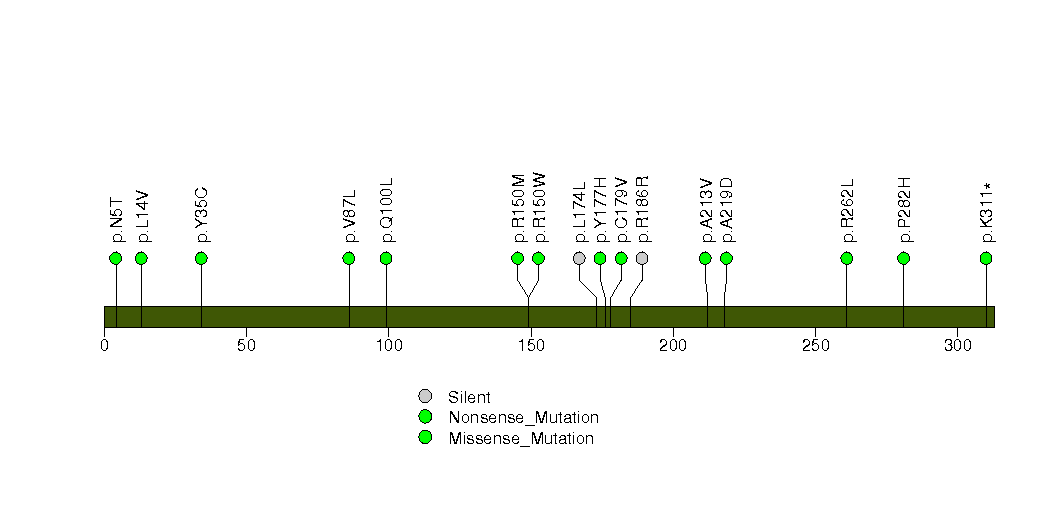
Figure S18. This figure depicts the distribution of mutations and mutation types across the ASB5 significant gene.
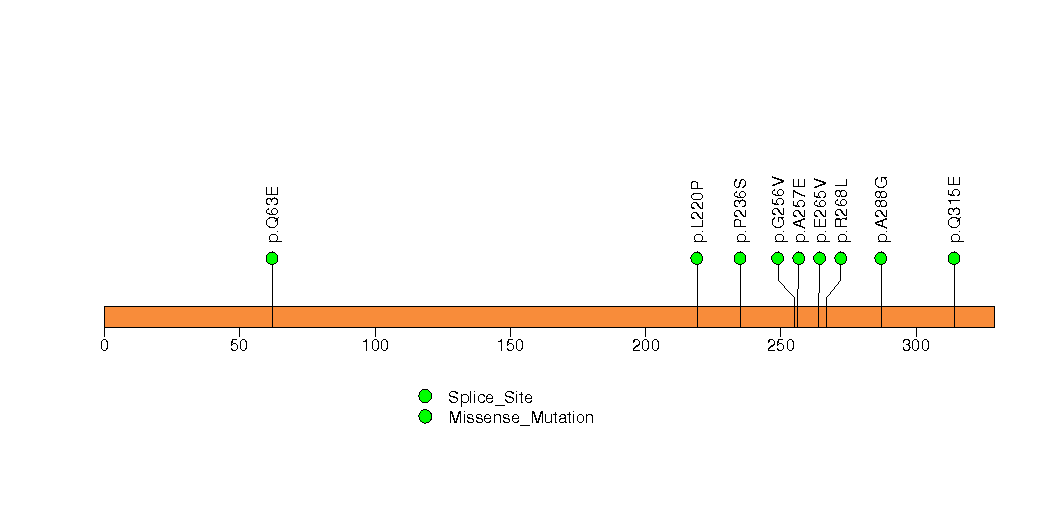
Figure S19. This figure depicts the distribution of mutations and mutation types across the CYP11B1 significant gene.

Figure S20. This figure depicts the distribution of mutations and mutation types across the CRB1 significant gene.
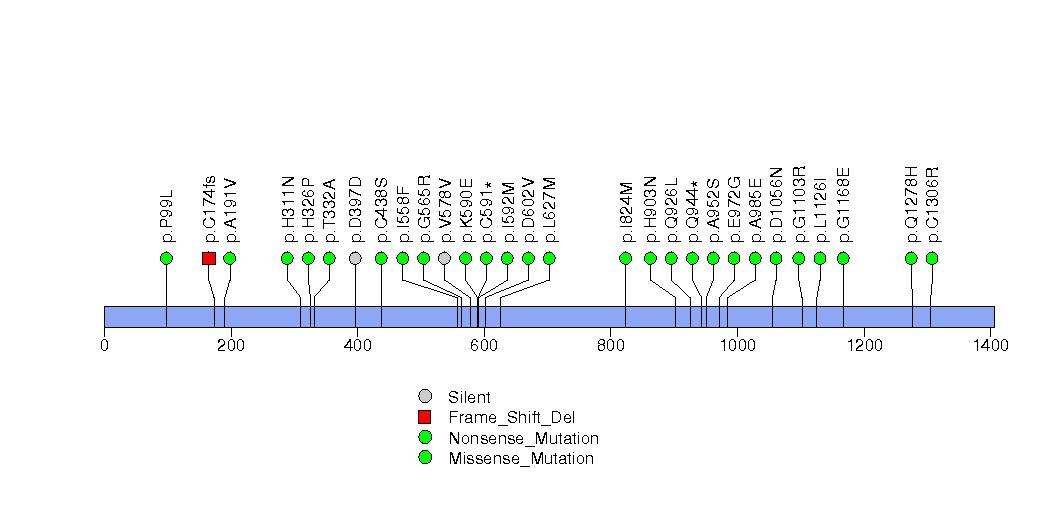
Figure S21. This figure depicts the distribution of mutations and mutation types across the OR6F1 significant gene.

Figure S22. This figure depicts the distribution of mutations and mutation types across the CPS1 significant gene.
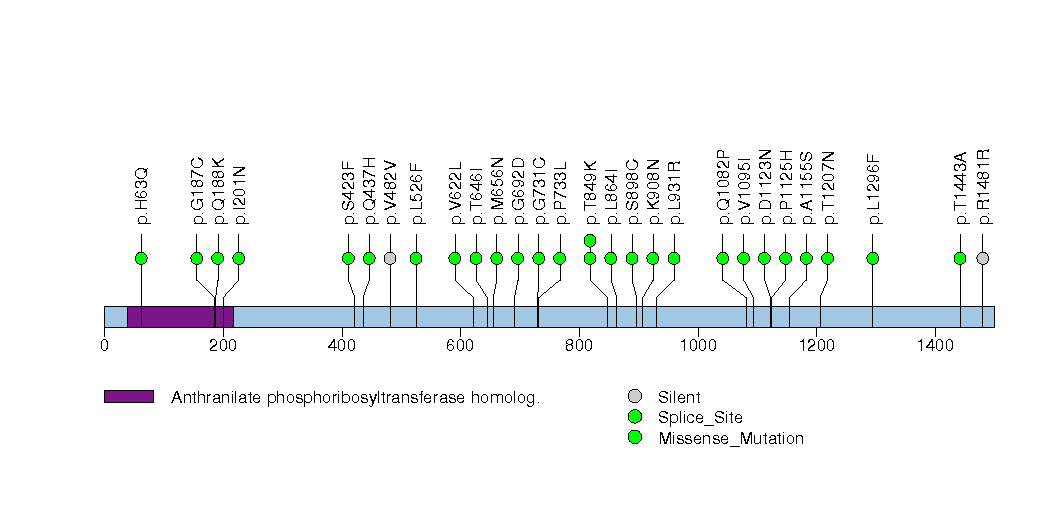
Figure S23. This figure depicts the distribution of mutations and mutation types across the OR2T33 significant gene.

Figure S24. This figure depicts the distribution of mutations and mutation types across the ESRRG significant gene.

Figure S25. This figure depicts the distribution of mutations and mutation types across the REG3A significant gene.

Figure S26. This figure depicts the distribution of mutations and mutation types across the PNLIPRP3 significant gene.

Figure S27. This figure depicts the distribution of mutations and mutation types across the SPHKAP significant gene.

Figure S28. This figure depicts the distribution of mutations and mutation types across the COL22A1 significant gene.

Figure S29. This figure depicts the distribution of mutations and mutation types across the MAGEB2 significant gene.

Figure S30. This figure depicts the distribution of mutations and mutation types across the SLC13A1 significant gene.

Figure S31. This figure depicts the distribution of mutations and mutation types across the OR51B2 significant gene.

Figure S32. This figure depicts the distribution of mutations and mutation types across the TGIF2LX significant gene.
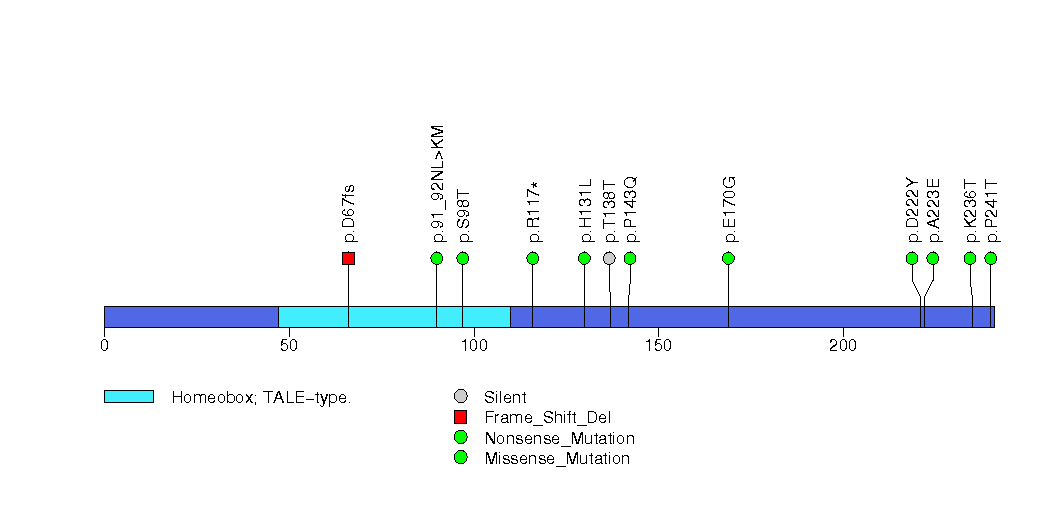
Figure S33. This figure depicts the distribution of mutations and mutation types across the USP29 significant gene.
In this analysis, COSMIC is used as a filter to increase power by restricting the territory of each gene. Cosmic version: v48.
Table 4. Get Full Table Significantly mutated genes (COSMIC territory only). To access the database please go to: COSMIC. Number of significant genes found: 11. Number of genes displayed: 10
| rank | gene | description | n | cos | n_cos | N_cos | cos_ev | p | q |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TP53 | tumor protein p53 | 149 | 356 | 146 | 63368 | 25884 | 0 | 0 |
| 2 | PIK3CA | phosphoinositide-3-kinase, catalytic, alpha polypeptide | 29 | 220 | 24 | 39160 | 9572 | 0 | 0 |
| 3 | CDKN2A | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | 26 | 332 | 25 | 59096 | 559 | 0 | 0 |
| 4 | PTEN | phosphatase and tensin homolog (mutated in multiple advanced cancers 1) | 16 | 767 | 16 | 136526 | 579 | 0 | 0 |
| 5 | HRAS | v-Ha-ras Harvey rat sarcoma viral oncogene homolog | 5 | 19 | 5 | 3382 | 1133 | 1.7e-10 | 1.5e-07 |
| 6 | RB1 | retinoblastoma 1 (including osteosarcoma) | 12 | 267 | 7 | 47526 | 17 | 2.7e-07 | 0.0002 |
| 7 | FBXW7 | F-box and WD repeat domain containing 7 | 10 | 91 | 5 | 16198 | 217 | 3.9e-07 | 0.00025 |
| 8 | HEPACAM2 | HEPACAM family member 2 | 4 | 1 | 2 | 178 | 2 | 1.2e-06 | 0.00066 |
| 9 | NF1 | neurofibromin 1 (neurofibromatosis, von Recklinghausen disease, Watson disease) | 22 | 285 | 6 | 50730 | 21 | 6.7e-06 | 0.0034 |
| 10 | BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 8 | 89 | 4 | 15842 | 77 | 0.000013 | 0.0059 |
Note:
n - number of (nonsilent) mutations in this gene across the individual set.
cos = number of unique mutated sites in this gene in COSMIC
n_cos = overlap between n and cos.
N_cos = number of individuals times cos.
cos_ev = total evidence: number of reports in COSMIC for mutations seen in this gene.
p = p-value for seeing the observed amount of overlap in this gene)
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 5. Get Full Table A Ranked List of Significantly Mutated Genesets. (Source: MSigDB GSEA Cannonical Pathway Set).Number of significant genesets found: 19. Number of genesets displayed: 10
| rank | geneset | description | genes | N_genes | mut_tally | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_ns_s | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | G1PATHWAY | CDK4/6-cyclin D and CDK2-cyclin E phosphorylate Rb, which allows the transcription of genes needed for the G1/S cell cycle transition. | ABL1, ATM, ATR, CCNA1, CCND1, CCNE1, CDC2, CDC25A, CDK2, CDK4, CDK6, CDKN1A, CDKN1B, CDKN2A, CDKN2B, DHFR, E2F1, GSK3B, HDAC1, MADH3, MADH4, RB1, SKP2, TFDP1, TGFB1, TGFB2, TGFB3, TP53 | 25 | ABL1(3), ATM(8), ATR(12), CCNA1(1), CCND1(1), CCNE1(3), CDKN1A(2), CDKN2A(26), CDKN2B(1), E2F1(2), HDAC1(2), RB1(12), SKP2(2), TFDP1(3), TGFB2(3), TP53(149) | 7994705 | 230 | 158 | 175 | 11 | 28 | 62 | 20 | 39 | 80 | 1 | 1.5e-12 | <1.00e-15 | <2.05e-13 |
| 2 | TELPATHWAY | Telomerase is a ribonucleotide protein that adds telomeric repeats to the 3' ends of chromosomes. | AKT1, BCL2, EGFR, G22P1, HSPCA, IGF1R, KRAS2, MYC, POLR2A, PPP2CA, PRKCA, RB1, TEP1, TERF1, TERT, TNKS, TP53, XRCC5 | 15 | AKT1(1), EGFR(7), IGF1R(1), MYC(1), POLR2A(2), PRKCA(5), RB1(12), TEP1(7), TERF1(3), TERT(3), TNKS(7), TP53(149), XRCC5(2) | 7492174 | 200 | 153 | 147 | 11 | 23 | 51 | 17 | 42 | 66 | 1 | 9.1e-12 | 1.11e-15 | 2.05e-13 |
| 3 | SA_G1_AND_S_PHASES | Cdk2, 4, and 6 bind cyclin D in G1, while cdk2/cyclin E promotes the G1/S transition. | ARF1, ARF3, CCND1, CDK2, CDK4, CDKN1A, CDKN1B, CDKN2A, CFL1, E2F1, E2F2, MDM2, NXT1, PRB1, TP53 | 15 | ARF1(1), CCND1(1), CDKN1A(2), CDKN2A(26), CFL1(2), E2F1(2), MDM2(2), NXT1(1), PRB1(5), TP53(149) | 2143952 | 191 | 152 | 136 | 6 | 17 | 52 | 17 | 37 | 67 | 1 | 1.4e-14 | 1.11e-15 | 2.05e-13 |
| 4 | PMLPATHWAY | Ring-shaped PML nuclear bodies regulate transcription and are required co-activators in p53- and DAXX-mediated apoptosis. | CREBBP, DAXX, HRAS, PAX3, PML, PRAM-1, RARA, RB1, SIRT1, SP100, TNF, TNFRSF1A, TNFRSF1B, TNFRSF6, TNFSF6, TP53, UBL1 | 13 | CREBBP(17), DAXX(3), HRAS(5), PAX3(3), RARA(1), RB1(12), SIRT1(1), SP100(3), TNF(1), TNFRSF1A(2), TNFRSF1B(1), TP53(149) | 5020302 | 198 | 150 | 145 | 11 | 16 | 54 | 20 | 42 | 65 | 1 | 6.8e-11 | 1.55e-15 | 2.05e-13 |
| 5 | P53PATHWAY | p53 induces cell cycle arrest or apoptosis under conditions of DNA damage. | APAF1, ATM, BAX, BCL2, CCND1, CCNE1, CDK2, CDK4, CDKN1A, E2F1, GADD45A, MDM2, PCNA, RB1, TIMP3, TP53 | 16 | APAF1(4), ATM(8), BAX(1), CCND1(1), CCNE1(3), CDKN1A(2), E2F1(2), GADD45A(1), MDM2(2), RB1(12), TP53(149) | 4817072 | 185 | 152 | 133 | 2 | 16 | 49 | 17 | 38 | 64 | 1 | 2.3e-15 | 2.00e-15 | 2.05e-13 |
| 6 | CHEMICALPATHWAY | DNA damage promotes Bid cleavage, which stimulates mitochondrial cytochrome c release and consequent caspase activation, resulting in apoptosis. | ADPRT, AKT1, APAF1, ATM, BAD, BAX, BCL2, BCL2L1, BID, CASP3, CASP6, CASP7, CASP9, CYCS, EIF2S1, PRKCA, PRKCB1, PTK2, PXN, STAT1, TLN1, TP53 | 20 | AKT1(1), APAF1(4), ATM(8), BAX(1), BCL2L1(1), CASP7(2), CASP9(3), PRKCA(5), PTK2(6), PXN(3), STAT1(2), TLN1(6), TP53(149) | 7279788 | 191 | 152 | 138 | 12 | 24 | 52 | 20 | 40 | 54 | 1 | 4.4e-09 | 2.11e-15 | 2.05e-13 |
| 7 | RNAPATHWAY | dsRNA-activated protein kinase phosphorylates elF2a, which generally inhibits translation, and activates NF-kB to provoke inflammation. | CHUK, DNAJC3, EIF2S1, EIF2S2, MAP3K14, NFKB1, NFKBIA, PRKR, RELA, TP53 | 9 | CHUK(2), EIF2S2(1), NFKB1(2), RELA(3), TP53(149) | 2611540 | 157 | 145 | 105 | 3 | 10 | 41 | 17 | 34 | 54 | 1 | 1.8e-12 | 3.00e-15 | 2.05e-13 |
| 8 | ARFPATHWAY | Cyclin-dependent kinase inhibitor 2A is a tumor suppressor that induces G1 arrest and can activate the p53 pathway, leading to G2/M arrest. | ABL1, CDKN2A, E2F1, MDM2, MYC, PIK3CA, PIK3R1, POLR1A, POLR1B, POLR1C, POLR1D, RAC1, RB1, TBX2, TP53, TWIST1 | 16 | ABL1(3), CDKN2A(26), E2F1(2), MDM2(2), MYC(1), PIK3CA(29), PIK3R1(2), POLR1A(3), POLR1B(2), POLR1D(1), RAC1(1), RB1(12), TBX2(3), TP53(149), TWIST1(1) | 5361306 | 237 | 158 | 169 | 13 | 42 | 53 | 17 | 44 | 80 | 1 | 9.6e-13 | 3.11e-15 | 2.05e-13 |
| 9 | ATMPATHWAY | The tumor-suppressing protein kinase ATM responds to radiation-induced DNA damage by blocking cell-cycle progression and activating DNA repair. | ABL1, ATM, BRCA1, CDKN1A, CHEK1, CHEK2, GADD45A, JUN, MAPK8, MDM2, MRE11A, NBS1, NFKB1, NFKBIA, RAD50, RAD51, RBBP8, RELA, TP53, TP73 | 19 | ABL1(3), ATM(8), BRCA1(12), CDKN1A(2), CHEK1(5), CHEK2(3), GADD45A(1), JUN(3), MAPK8(2), MDM2(2), MRE11A(1), NFKB1(2), RAD50(3), RAD51(1), RBBP8(1), RELA(3), TP53(149) | 7908840 | 201 | 147 | 148 | 9 | 27 | 53 | 18 | 42 | 60 | 1 | 1.1e-10 | 3.33e-15 | 2.05e-13 |
| 10 | TERTPATHWAY | hTERC, the RNA subunit of telomerase, and hTERT, the catalytic protein subunit, are required for telomerase activity and are overexpressed in many cancers. | HDAC1, MAX, MYC, SP1, SP3, TP53, WT1, ZNF42 | 7 | HDAC1(2), MYC(1), SP1(2), SP3(1), TP53(149), WT1(4) | 1863398 | 159 | 149 | 107 | 6 | 9 | 43 | 18 | 36 | 52 | 1 | 2e-10 | 3.44e-15 | 2.05e-13 |
Table 6. Get Full Table A Ranked List of Significantly Mutated Genesets (Excluding Significantly Mutated Genes). Number of significant genesets found: 0. Number of genesets displayed: 10
| rank | geneset | description | genes | N_genes | mut_tally | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_ns_s | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HSA00627_1,4_DICHLOROBENZENE_DEGRADATION | Genes involved in 1,4-dichlorobenzene degradation | CMBL | 1 | CMBL(3) | 134908 | 3 | 3 | 3 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0.81 | 0.033 | 1 |
| 2 | SA_G2_AND_M_PHASES | Cdc25 activates the cdc2/cyclin B complex to induce the G2/M transition. | CDC2, CDC25A, CDC25B, CDK7, CDKN1A, CHEK1, NEK1, WEE1 | 7 | CDC25B(2), CDK7(1), CDKN1A(2), CHEK1(5), NEK1(3), WEE1(4) | 1849805 | 17 | 17 | 17 | 1 | 7 | 5 | 1 | 3 | 1 | 0 | 0.1 | 0.17 | 1 |
| 3 | HSA00750_VITAMIN_B6_METABOLISM | Genes involved in vitamin B6 metabolism | AOX1, PDXK, PDXP, PNPO, PSAT1 | 5 | AOX1(13), PDXP(1), PSAT1(3) | 1279971 | 17 | 16 | 17 | 2 | 6 | 5 | 0 | 5 | 1 | 0 | 0.13 | 0.21 | 1 |
| 4 | HSA00902_MONOTERPENOID_BIOSYNTHESIS | Genes involved in monoterpenoid biosynthesis | CYP2C19, CYP2C9 | 2 | CYP2C19(7), CYP2C9(3) | 536360 | 10 | 9 | 10 | 3 | 5 | 3 | 0 | 1 | 1 | 0 | 0.6 | 0.28 | 1 |
| 5 | HSA00550_PEPTIDOGLYCAN_BIOSYNTHESIS | Genes involved in peptidoglycan biosynthesis | GLUL, PGLYRP2 | 2 | GLUL(4), PGLYRP2(2) | 477868 | 6 | 5 | 6 | 0 | 1 | 4 | 1 | 0 | 0 | 0 | 0.15 | 0.31 | 1 |
| 6 | HSA00472_D_ARGININE_AND_D_ORNITHINE_METABOLISM | Genes involved in D-arginine and D-ornithine metabolism | DAO | 1 | DAO(2) | 191569 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0.5 | 0.34 | 1 |
| 7 | P27PATHWAY | p27 blocks the G1/S transition by inhibiting the checkpoint kinase cdk2/cyclin E and is inhibited by cdk2-mediated ubiquitination. | CCNE1, CDK2, CDKN1B, CKS1B, CUL1, E2F1, NEDD8, RB1, RBX1, SKP1A, SKP2, TFDP1, UBE2M | 11 | CCNE1(3), CKS1B(1), CUL1(4), E2F1(2), RBX1(1), SKP2(2), TFDP1(3), UBE2M(1) | 1813849 | 17 | 16 | 17 | 1 | 5 | 8 | 0 | 2 | 2 | 0 | 0.05 | 0.37 | 1 |
| 8 | HSA00780_BIOTIN_METABOLISM | Genes involved in biotin metabolism | BTD, HLCS, SPCS1, SPCS3 | 4 | BTD(2), HLCS(5), SPCS1(1), SPCS3(2) | 816418 | 10 | 9 | 10 | 2 | 2 | 2 | 2 | 0 | 4 | 0 | 0.4 | 0.44 | 1 |
| 9 | IL18PATHWAY | Pro-inflammatory IL-18 is activated in macrophages by caspase-1 cleavage and, in conjunction with IL-12, stimulates Th1 cell differentiation. | CASP1, IFNG, IL12A, IL12B, IL18, IL2 | 6 | CASP1(1), IFNG(1), IL12A(1), IL12B(3) | 791855 | 6 | 6 | 6 | 1 | 3 | 1 | 0 | 0 | 2 | 0 | 0.52 | 0.49 | 1 |
| 10 | BLOOD_GROUP_GLYCOLIPID_BIOSYNTHESIS_LACTOSERIES | ABO, FUT1, FUT2, FUT3, FUT5, FUT6, SIAT6, ST3GAL3 | 7 | FUT2(1), FUT3(3), FUT5(5), FUT6(2) | 1347433 | 11 | 10 | 11 | 1 | 0 | 5 | 4 | 1 | 1 | 0 | 0.1 | 0.49 | 1 |
In brief, we tabulate the number of mutations and the number of covered bases for each gene. The counts are broken down by mutation context category: four context categories that are discovered by MutSig, and one for indel and 'null' mutations, which include indels, nonsense mutations, splice-site mutations, and non-stop (read-through) mutations. For each gene, we calculate the probability of seeing the observed constellation of mutations, i.e. the product P1 x P2 x ... x Pm, or a more extreme one, given the background mutation rates calculated across the dataset. [1]
In addition to the links below, the full results of the analysis summarized in this report can also be downloaded programmatically using firehose_get, or interactively from either the Broad GDAC website or TCGA Data Coordination Center Portal.