(BRAF_Hotspot_Mutants cohort)
This report serves to describe the mutational landscape and properties of a given individual set, as well as rank genes and genesets according to mutational significance. MutSig v2.0 was used to generate the results found in this report.
-
Working with individual set: SKCM-BRAF_Hotspot_Mutants
-
Number of patients in set: 97
The input for this pipeline is a set of individuals with the following files associated for each:
-
An annotated .maf file describing the mutations called for the respective individual, and their properties.
-
A .wig file that contains information about the coverage of the sample.
-
MAF used for this analysis:SKCM-BRAF_Hotspot_Mutants.final_analysis_set.maf
-
Significantly mutated genes (q ≤ 0.1): 58
-
Mutations seen in COSMIC: 232
-
Significantly mutated genes in COSMIC territory: 10
-
Genes with clustered mutations (≤ 3 aa apart): 899
-
Significantly mutated genesets: 2
-
Significantly mutated genesets: (excluding sig. mutated genes):0
-
Read 97 MAFs of type "Broad"
-
Total number of mutations in input MAFs: 55227
-
After removing 2 mutations outside chr1-24: 55225
-
After removing 325 blacklisted mutations: 54900
-
After removing 1075 noncoding mutations: 53825
-
Number of mutations before filtering: 53825
-
After removing 723 mutations outside gene set: 53102
-
After removing 32 mutations outside category set: 53070
Table 1. Get Full Table Table representing breakdown of mutations by type.
| type | count |
|---|---|
| Frame_Shift_Del | 304 |
| Frame_Shift_Ins | 94 |
| In_Frame_Del | 105 |
| In_Frame_Ins | 13 |
| Missense_Mutation | 33009 |
| Nonsense_Mutation | 1986 |
| Nonstop_Mutation | 14 |
| Silent | 16955 |
| Splice_Site | 575 |
| Translation_Start_Site | 15 |
| Total | 53070 |
Table 2. Get Full Table A breakdown of mutation rates per category discovered for this individual set.
| category | n | N | rate | rate_per_mb | relative_rate | exp_ns_s_ratio |
|---|---|---|---|---|---|---|
| (C/T)p*C->T | 23000 | 771897641 | 3e-05 | 30 | 2.3 | 1.6 |
| (A/G)p*C->T | 2721 | 647491941 | 4.2e-06 | 4.2 | 0.32 | 1.9 |
| A->G | 1765 | 1373132947 | 1.3e-06 | 1.3 | 0.099 | 2.3 |
| transver | 5538 | 2792522529 | 2e-06 | 2 | 0.15 | 5 |
| indel+null | 3060 | 2792522529 | 1.1e-06 | 1.1 | 0.085 | NaN |
| double_null | 31 | 2792522529 | 1.1e-08 | 0.011 | 0.00086 | NaN |
| Total | 36115 | 2792522529 | 0.000013 | 13 | 1 | 3.5 |
The x axis represents the samples. The y axis represents the exons, one row per exon, and they are sorted by average coverage across samples. For exons with exactly the same average coverage, they are sorted next by the %GC of the exon. (The secondary sort is especially useful for the zero-coverage exons at the bottom).
Figure 1.

Figure 2. Patients counts and rates file used to generate this plot: SKCM-BRAF_Hotspot_Mutants.patients.counts_and_rates.txt
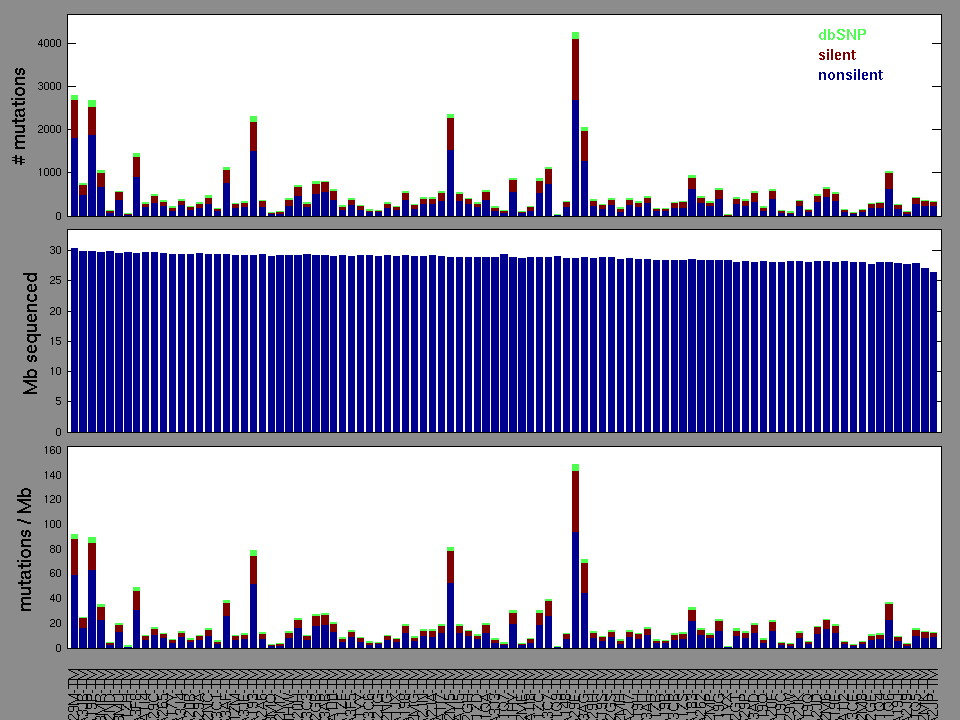
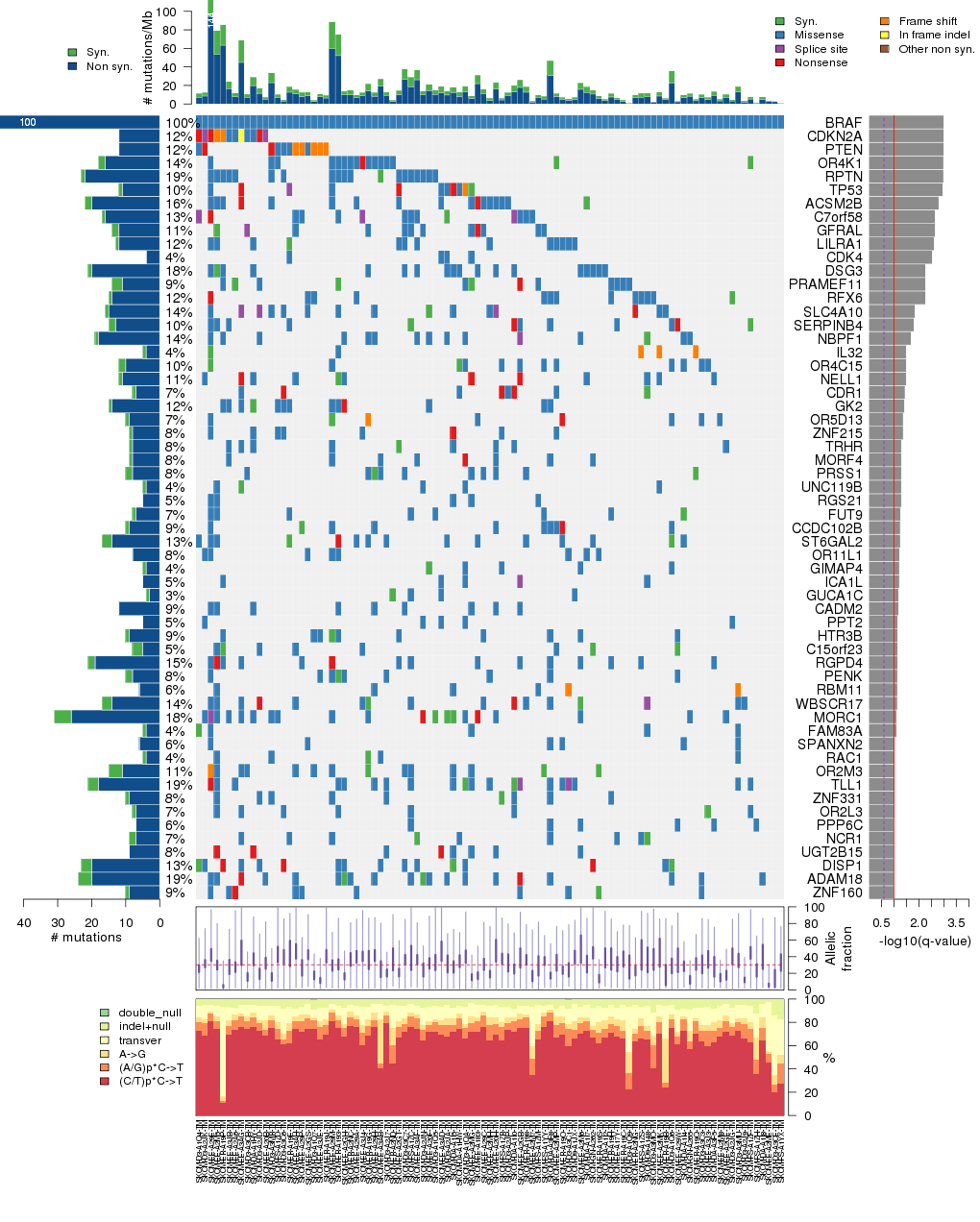
Figure 3. Get High-res Image The matrix in the center of the figure represents individual mutations in patient samples, color-coded by type of mutation, for the significantly mutated genes. The rate of synonymous and non-synonymous mutations is displayed at the top of the matrix. The barplot on the left of the matrix shows the number of mutations in each gene. The percentages represent the fraction of tumors with at least one mutation in the specified gene. The barplot to the right of the matrix displays the q-values for the most significantly mutated genes. The purple boxplots below the matrix (only displayed if required columns are present in the provided MAF) represent the distributions of allelic fractions observed in each sample. The plot at the bottom represents the base substitution distribution of individual samples, using the same categories that were used to calculate significance.
Column Descriptions:
-
N = number of sequenced bases in this gene across the individual set
-
n = number of (nonsilent) mutations in this gene across the individual set
-
npat = number of patients (individuals) with at least one nonsilent mutation
-
nsite = number of unique sites having a non-silent mutation
-
nsil = number of silent mutations in this gene across the individual set
-
n1 = number of nonsilent mutations of type: (C/T)p*C->T
-
n2 = number of nonsilent mutations of type: (A/G)p*C->T
-
n3 = number of nonsilent mutations of type: A->G
-
n4 = number of nonsilent mutations of type: transver
-
n5 = number of nonsilent mutations of type: indel+null
-
n6 = number of nonsilent mutations of type: double_null
-
p_classic = p-value for the observed amount of nonsilent mutations being elevated in this gene
-
p_ns_s = p-value for the observed nonsilent/silent ratio being elevated in this gene
-
p_cons = p-value for enrichment of mutations at evolutionarily most-conserved sites in gene
-
p_joint = p-value for clustering + conservation
-
p = p-value (overall)
-
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 3. Get Full Table A Ranked List of Significantly Mutated Genes. Number of significant genes found: 58. Number of genes displayed: 35. Click on a gene name to display its stick figure depicting the distribution of mutations and mutation types across the chosen gene (this feature may not be available for all significant genes).
| rank | gene | description | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_classic | p_ns_s | p_cons | p_joint | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 215475 | 99 | 97 | 4 | 1 | 2 | 0 | 0 | 97 | 0 | 0 | <1.00e-15 | 1.5e-07 | 0 | 0 | <1.00e-15 | <1.80e-11 |
| 2 | CDKN2A | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | 89548 | 12 | 12 | 10 | 0 | 4 | 0 | 0 | 0 | 8 | 0 | 3.83e-10 | 0.053 | 0.000035 | 0.000037 | 4.62e-13 | 4.17e-09 |
| 3 | PTEN | phosphatase and tensin homolog (mutated in multiple advanced cancers 1) | 108063 | 12 | 12 | 11 | 0 | 1 | 0 | 3 | 1 | 7 | 0 | 4.08e-13 | 0.089 | 0.74 | 0.26 | 3.24e-12 | 1.95e-08 |
| 4 | OR4K1 | olfactory receptor, family 4, subfamily K, member 1 | 91018 | 16 | 14 | 13 | 2 | 14 | 1 | 0 | 0 | 1 | 0 | 3.30e-10 | 0.0064 | 0.78 | 0.89 | 6.76e-09 | 2.74e-05 |
| 5 | RPTN | repetin | 229211 | 22 | 18 | 21 | 1 | 13 | 1 | 4 | 4 | 0 | 0 | 6.78e-10 | 0.011 | 0.73 | 0.49 | 7.60e-09 | 2.74e-05 |
| 6 | TP53 | tumor protein p53 | 118128 | 11 | 10 | 11 | 1 | 2 | 0 | 2 | 2 | 5 | 0 | 3.05e-08 | 0.094 | 0.064 | 0.019 | 1.27e-08 | 3.82e-05 |
| 7 | ACSM2B | acyl-CoA synthetase medium-chain family member 2B | 168684 | 20 | 16 | 17 | 2 | 16 | 0 | 0 | 2 | 2 | 0 | 1.16e-08 | 0.0046 | 0.94 | 1 | 2.24e-07 | 0.000577 |
| 8 | C7orf58 | chromosome 7 open reading frame 58 | 301090 | 16 | 13 | 15 | 1 | 10 | 1 | 1 | 0 | 4 | 0 | 7.99e-06 | 0.011 | 0.001 | 0.0036 | 5.22e-07 | 0.00118 |
| 9 | GFRAL | GDNF family receptor alpha like | 116103 | 12 | 11 | 12 | 2 | 7 | 1 | 0 | 2 | 2 | 0 | 5.58e-08 | 0.18 | 0.39 | 0.58 | 5.93e-07 | 0.00119 |
| 10 | LILRA1 | leukocyte immunoglobulin-like receptor, subfamily A (with TM domain), member 1 | 145978 | 12 | 12 | 12 | 1 | 7 | 1 | 2 | 2 | 0 | 0 | 1.35e-07 | 0.024 | 0.39 | 0.33 | 7.98e-07 | 0.00144 |
| 11 | CDK4 | cyclin-dependent kinase 4 | 91043 | 4 | 4 | 3 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0.00337 | 0.42 | 0.032 | 0.000019 | 1.12e-06 | 0.00184 |
| 12 | DSG3 | desmoglein 3 (pemphigus vulgaris antigen) | 295650 | 20 | 17 | 18 | 1 | 18 | 2 | 0 | 0 | 0 | 0 | 4.20e-07 | 0.0042 | 0.69 | 0.4 | 2.77e-06 | 0.00416 |
| 13 | PRAMEF11 | PRAME family member 11 | 120600 | 11 | 9 | 8 | 3 | 3 | 0 | 3 | 4 | 1 | 0 | 1.89e-06 | 0.29 | 0.89 | 0.11 | 3.31e-06 | 0.00441 |
| 14 | RFX6 | regulatory factor X, 6 | 255411 | 14 | 12 | 14 | 1 | 11 | 0 | 1 | 1 | 1 | 0 | 2.06e-06 | 0.038 | 0.11 | 0.1 | 3.42e-06 | 0.00441 |
| 15 | SLC4A10 | solute carrier family 4, sodium bicarbonate transporter-like, member 10 | 266470 | 15 | 14 | 15 | 1 | 10 | 0 | 0 | 1 | 4 | 0 | 7.19e-07 | 0.029 | 0.93 | 1 | 1.09e-05 | 0.0131 |
| 16 | SERPINB4 | serpin peptidase inhibitor, clade B (ovalbumin), member 4 | 110945 | 13 | 10 | 13 | 2 | 10 | 0 | 0 | 1 | 2 | 0 | 1.28e-06 | 0.036 | 0.81 | 0.65 | 1.25e-05 | 0.0141 |
| 17 | NBPF1 | neuroblastoma breakpoint family, member 1 | 313745 | 18 | 14 | 16 | 1 | 9 | 1 | 1 | 6 | 1 | 0 | 1.56e-05 | 0.011 | 0.1 | 0.077 | 1.75e-05 | 0.0186 |
| 18 | IL32 | interleukin 32 | 48362 | 4 | 4 | 2 | 1 | 0 | 0 | 0 | 1 | 3 | 0 | 0.000187 | 0.93 | 0.86 | 0.011 | 2.90e-05 | 0.0290 |
| 19 | OR4C15 | olfactory receptor, family 4, subfamily C, member 15 | 107246 | 10 | 10 | 8 | 2 | 10 | 0 | 0 | 0 | 0 | 0 | 3.61e-06 | 0.055 | 0.75 | 0.64 | 3.20e-05 | 0.0296 |
| 20 | NELL1 | NEL-like 1 (chicken) | 232214 | 11 | 11 | 11 | 1 | 4 | 2 | 0 | 2 | 3 | 0 | 2.35e-06 | 0.1 | 0.92 | 1 | 3.28e-05 | 0.0296 |
| 21 | CDR1 | cerebellar degeneration-related protein 1, 34kDa | 76856 | 7 | 7 | 7 | 1 | 4 | 0 | 0 | 0 | 3 | 0 | 8.33e-06 | 0.26 | 0.68 | 0.35 | 4.03e-05 | 0.0346 |
| 22 | GK2 | glycerol kinase 2 | 160995 | 14 | 12 | 13 | 1 | 11 | 0 | 0 | 2 | 1 | 0 | 3.26e-06 | 0.057 | 0.58 | 1 | 4.44e-05 | 0.0365 |
| 23 | OR5D13 | olfactory receptor, family 5, subfamily D, member 13 | 90951 | 9 | 7 | 8 | 1 | 6 | 1 | 0 | 0 | 2 | 0 | 1.51e-05 | 0.034 | 0.91 | 0.26 | 5.27e-05 | 0.0412 |
| 24 | ZNF215 | zinc finger protein 215 | 152546 | 8 | 8 | 8 | 1 | 2 | 0 | 3 | 2 | 1 | 0 | 6.81e-06 | 0.2 | 0.8 | 0.6 | 5.49e-05 | 0.0412 |
| 25 | TRHR | thyrotropin-releasing hormone receptor | 115831 | 8 | 8 | 7 | 1 | 5 | 3 | 0 | 0 | 0 | 0 | 4.37e-05 | 0.045 | 0.32 | 0.13 | 7.30e-05 | 0.0488 |
| 26 | MORF4 | mortality factor 4 | 68955 | 8 | 8 | 8 | 1 | 6 | 0 | 0 | 1 | 1 | 0 | 5.65e-06 | 0.14 | 0.92 | 1 | 7.39e-05 | 0.0488 |
| 27 | PRSS1 | protease, serine, 1 (trypsin 1) | 74104 | 8 | 8 | 8 | 2 | 6 | 0 | 1 | 1 | 0 | 0 | 9.68e-06 | 0.18 | 0.64 | 0.6 | 7.55e-05 | 0.0488 |
| 28 | UNC119B | unc-119 homolog B (C. elegans) | 68908 | 4 | 4 | 4 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0.00385 | 0.6 | 0.0017 | 0.0015 | 7.57e-05 | 0.0488 |
| 29 | RGS21 | regulator of G-protein signaling 21 | 43410 | 5 | 5 | 5 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 7.85e-05 | 0.21 | 0.63 | 0.077 | 7.85e-05 | 0.0489 |
| 30 | FUT9 | fucosyltransferase 9 (alpha (1,3) fucosyltransferase) | 99525 | 7 | 7 | 7 | 1 | 4 | 1 | 1 | 1 | 0 | 0 | 5.09e-05 | 0.22 | 0.089 | 0.13 | 8.54e-05 | 0.0514 |
| 31 | CCDC102B | coiled-coil domain containing 102B | 148571 | 9 | 9 | 7 | 1 | 7 | 1 | 0 | 0 | 1 | 0 | 8.62e-05 | 0.11 | 0.6 | 0.086 | 9.53e-05 | 0.0543 |
| 32 | ST6GAL2 | ST6 beta-galactosamide alpha-2,6-sialyltranferase 2 | 138224 | 14 | 13 | 13 | 3 | 11 | 1 | 0 | 1 | 1 | 0 | 2.22e-05 | 0.068 | 0.087 | 0.34 | 9.62e-05 | 0.0543 |
| 33 | OR11L1 | olfactory receptor, family 11, subfamily L, member 1 | 93528 | 8 | 8 | 6 | 0 | 7 | 0 | 0 | 1 | 0 | 0 | 9.29e-05 | 0.0035 | 0.71 | 0.085 | 0.000101 | 0.0550 |
| 34 | GIMAP4 | GTPase, IMAP family member 4 | 96124 | 4 | 4 | 3 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0.0170 | 0.41 | 0.18 | 0.00052 | 0.000112 | 0.0592 |
| 35 | ICA1L | islet cell autoantigen 1,69kDa-like | 146534 | 5 | 5 | 3 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0.0120 | 0.46 | 0.0006 | 0.00076 | 0.000115 | 0.0592 |
Figure S1. This figure depicts the distribution of mutations and mutation types across the BRAF significant gene.
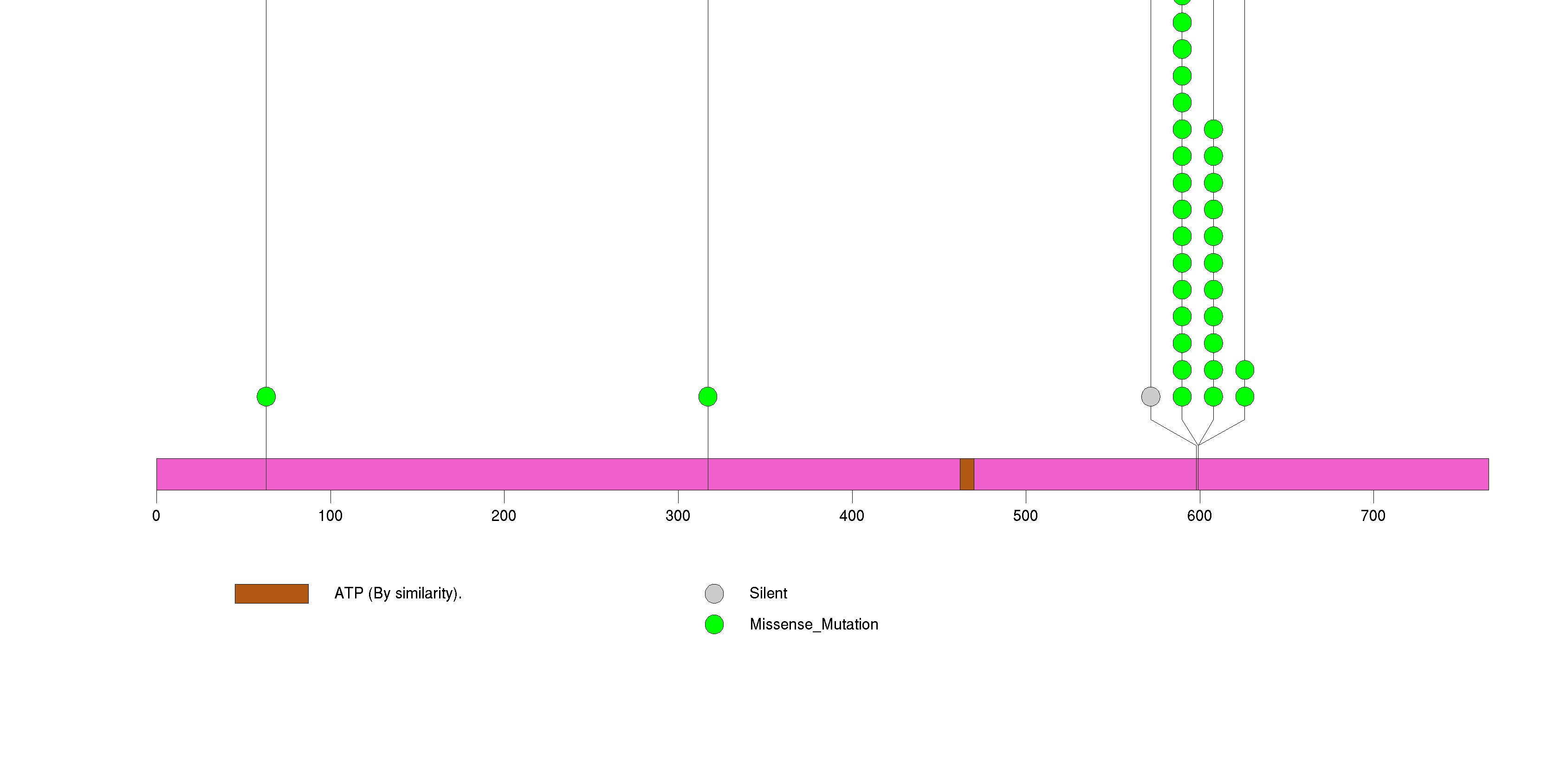
Figure S2. This figure depicts the distribution of mutations and mutation types across the CDKN2A significant gene.
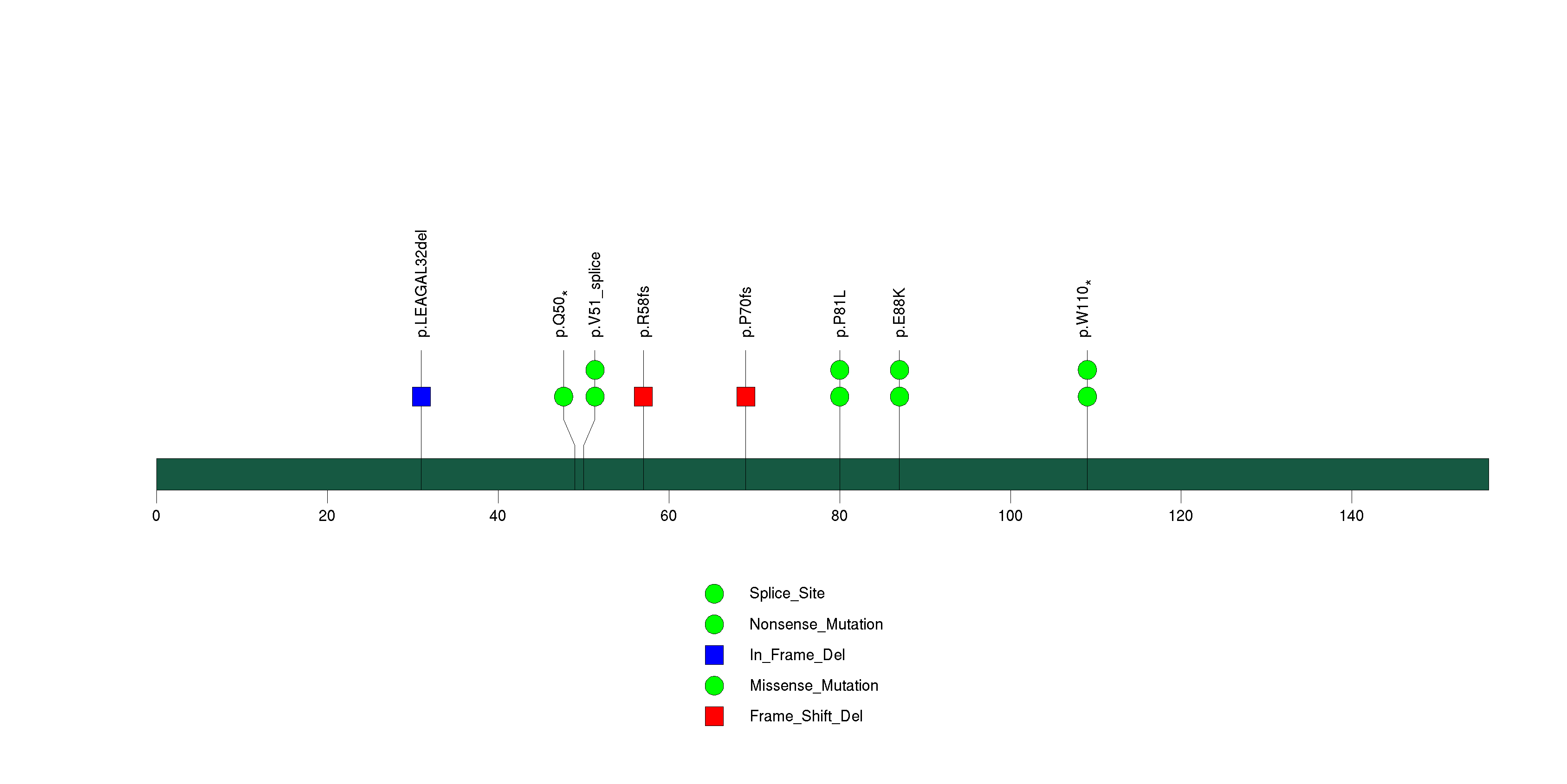
Figure S3. This figure depicts the distribution of mutations and mutation types across the PTEN significant gene.
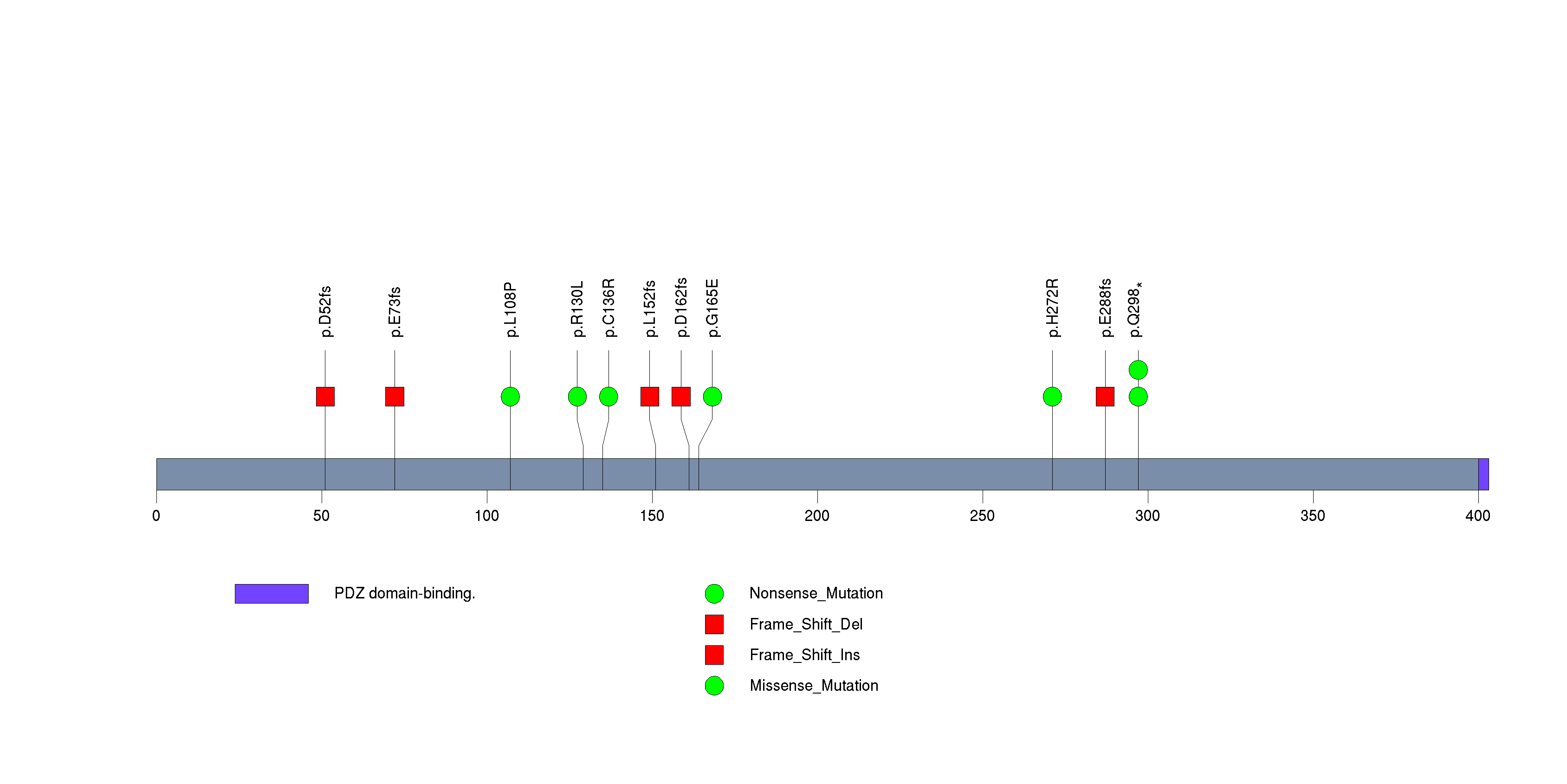
Figure S4. This figure depicts the distribution of mutations and mutation types across the OR4K1 significant gene.
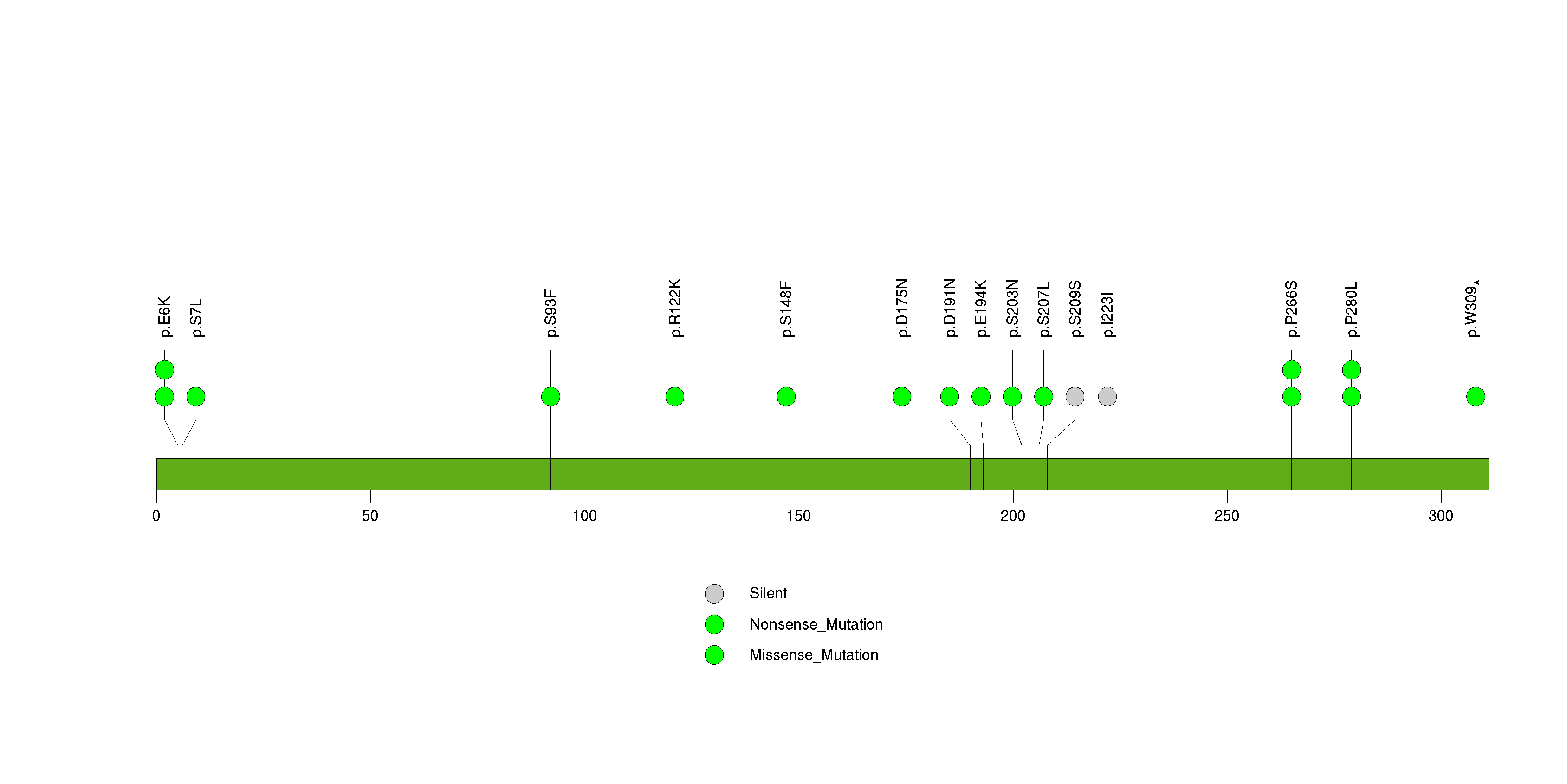
Figure S5. This figure depicts the distribution of mutations and mutation types across the RPTN significant gene.
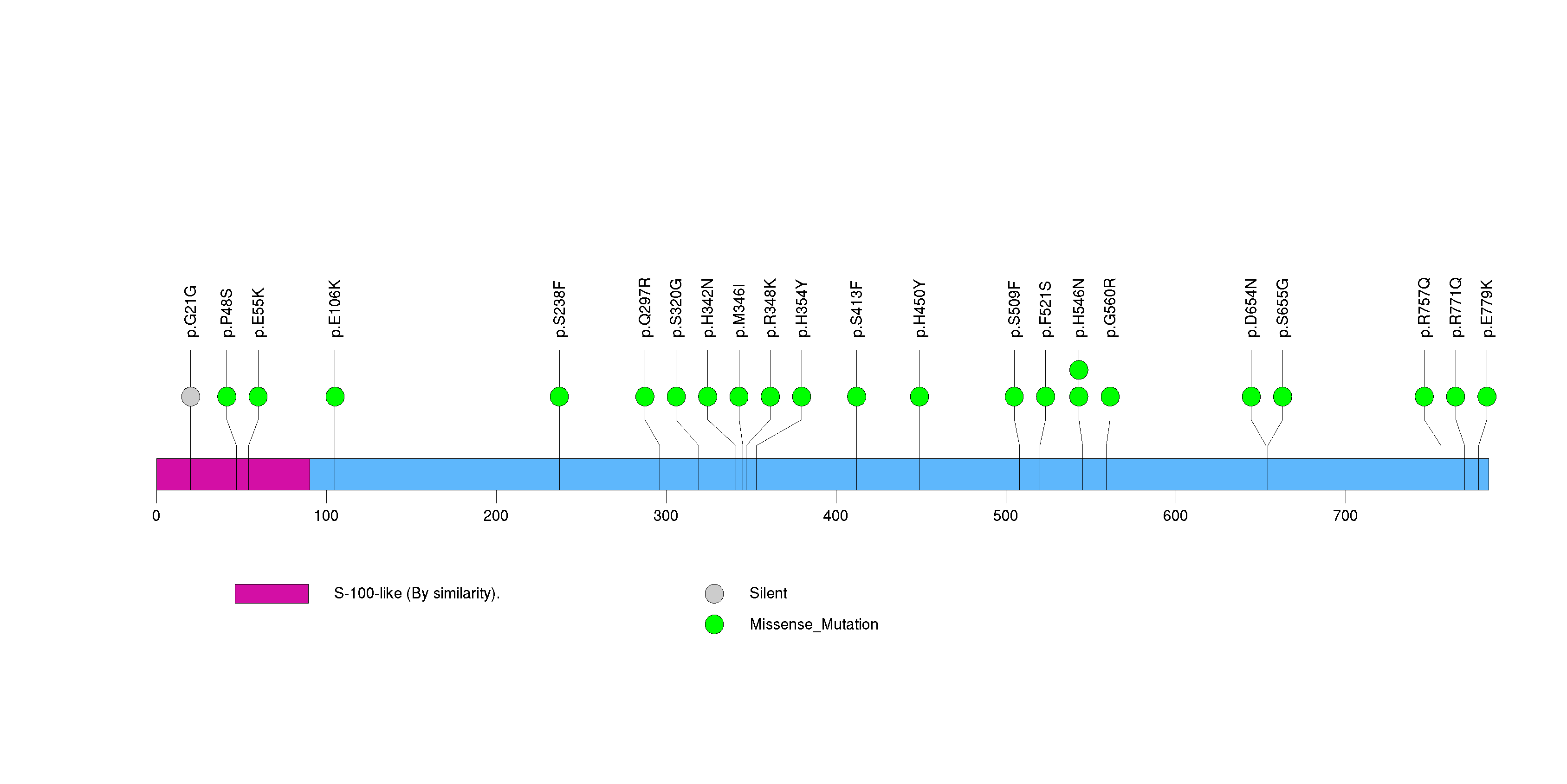
Figure S6. This figure depicts the distribution of mutations and mutation types across the TP53 significant gene.
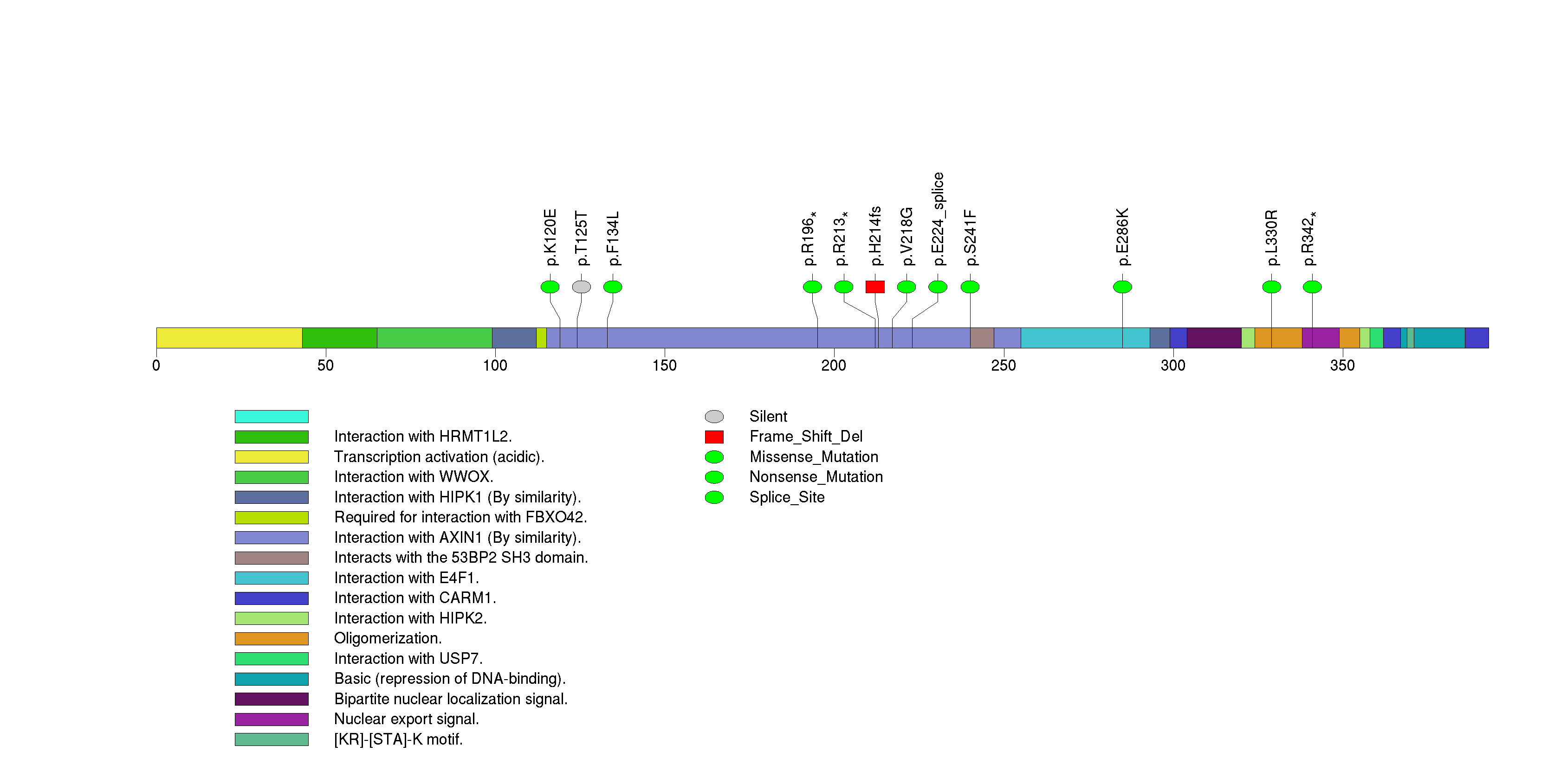
Figure S7. This figure depicts the distribution of mutations and mutation types across the ACSM2B significant gene.
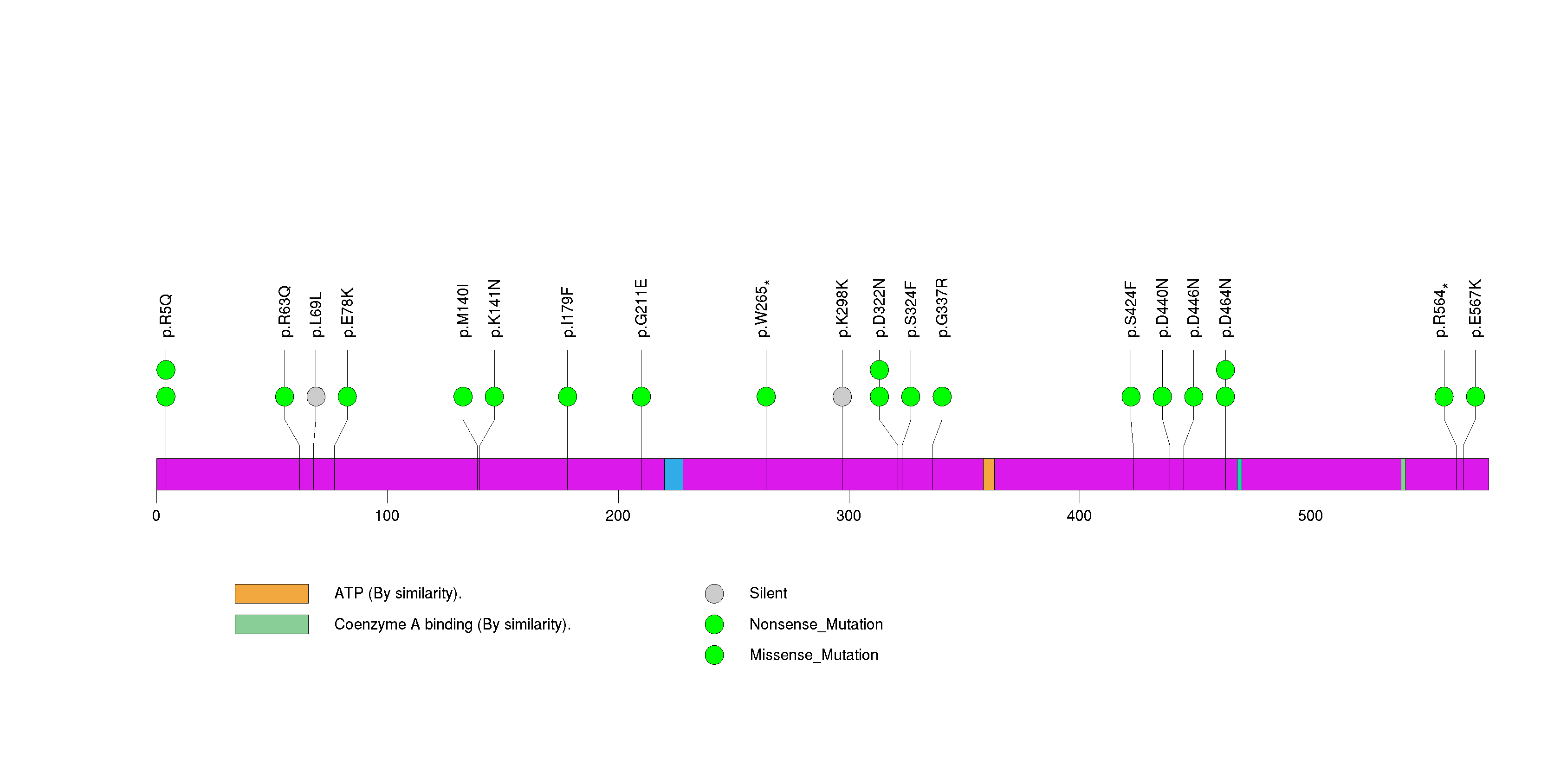
Figure S8. This figure depicts the distribution of mutations and mutation types across the C7orf58 significant gene.
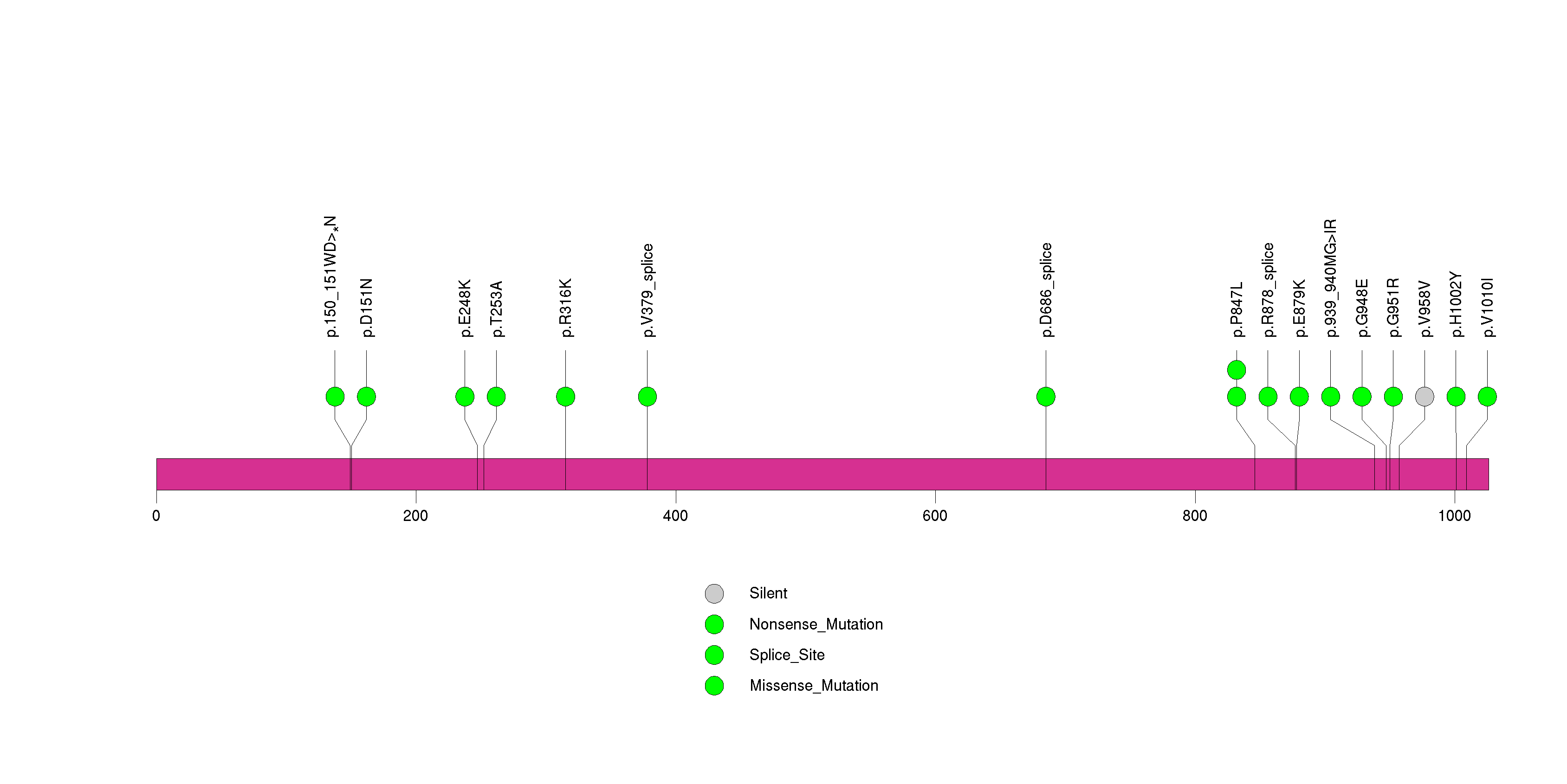
Figure S9. This figure depicts the distribution of mutations and mutation types across the GFRAL significant gene.
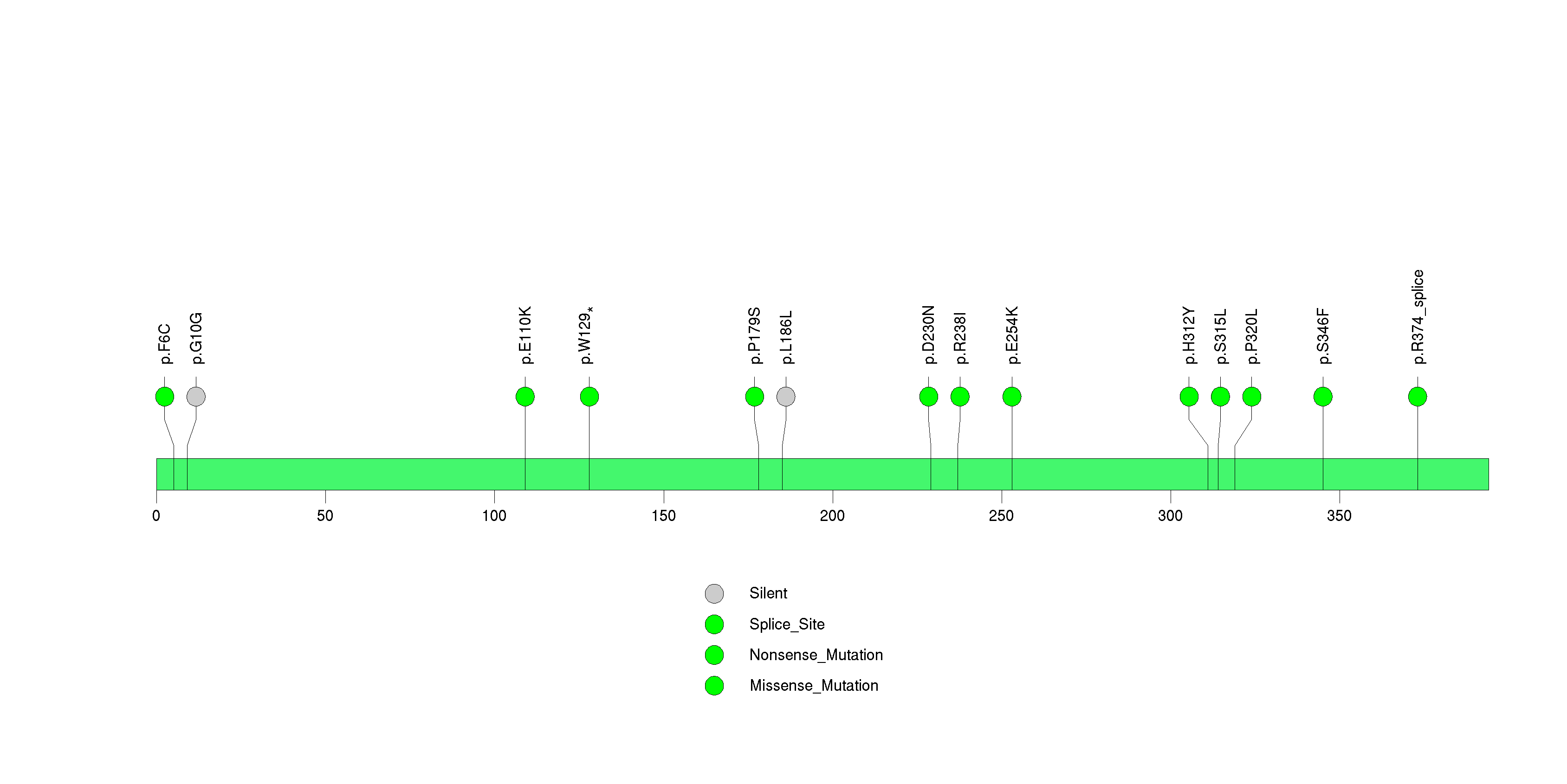
Figure S10. This figure depicts the distribution of mutations and mutation types across the LILRA1 significant gene.
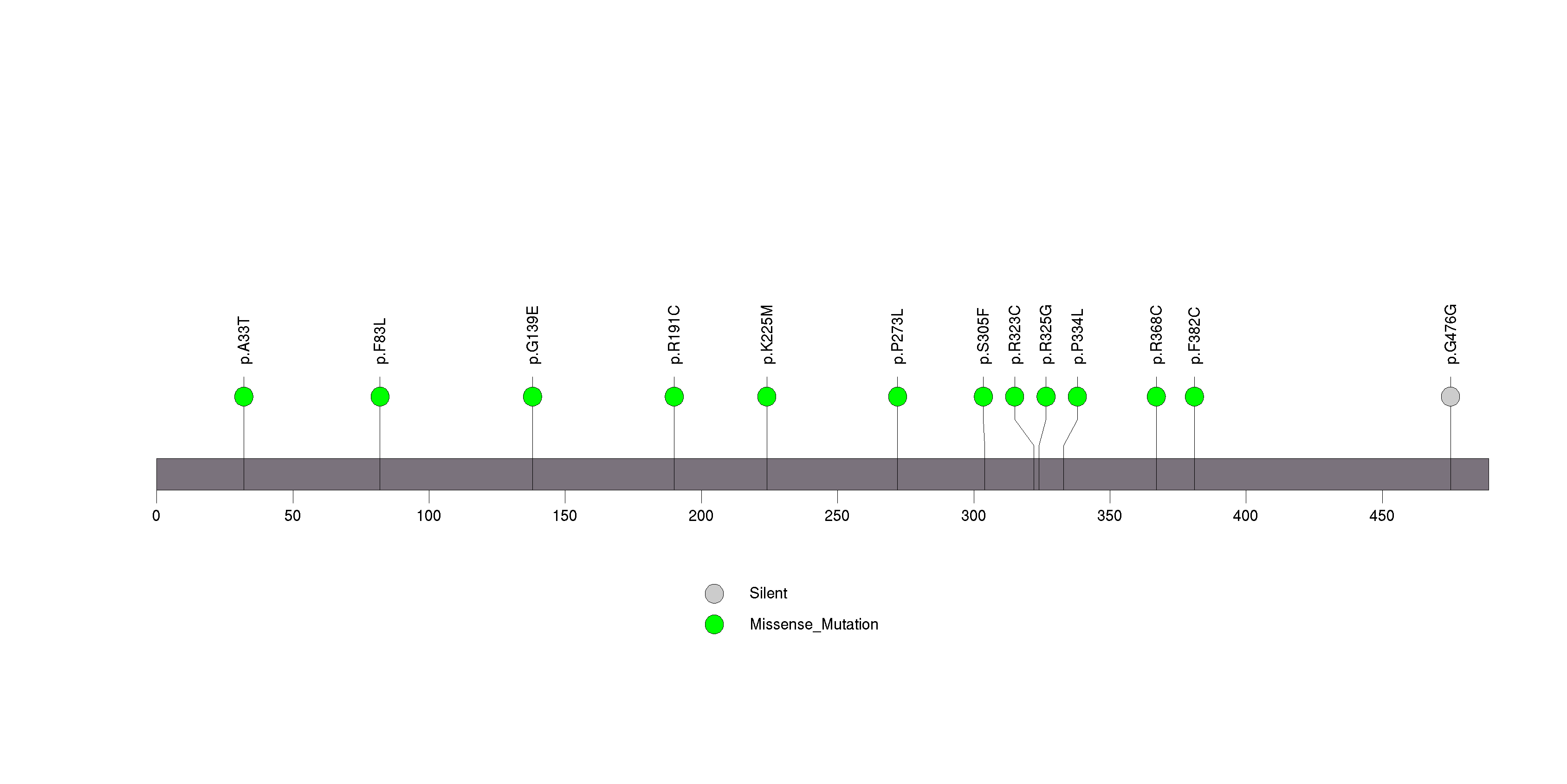
Figure S11. This figure depicts the distribution of mutations and mutation types across the CDK4 significant gene.
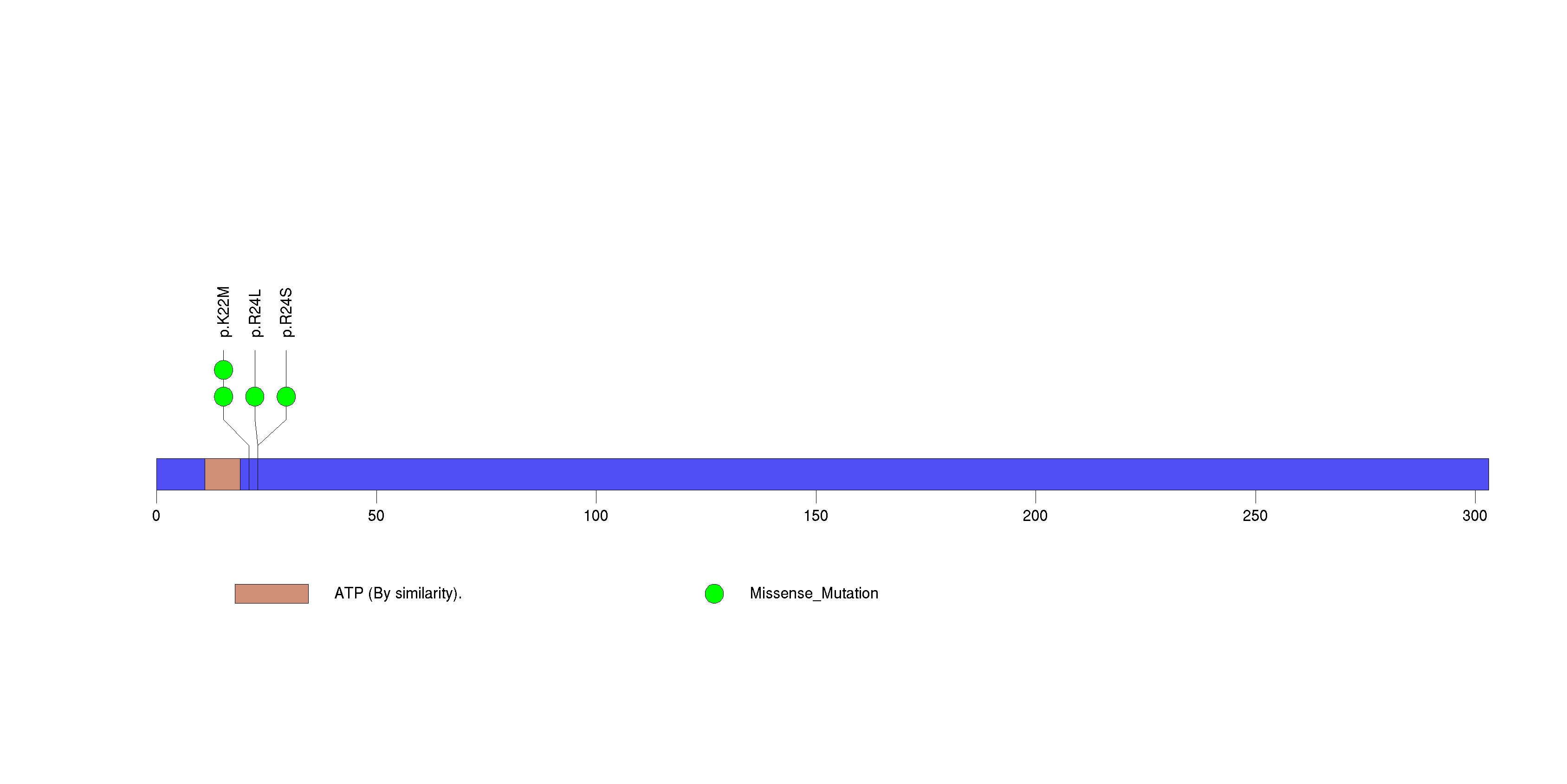
Figure S12. This figure depicts the distribution of mutations and mutation types across the DSG3 significant gene.
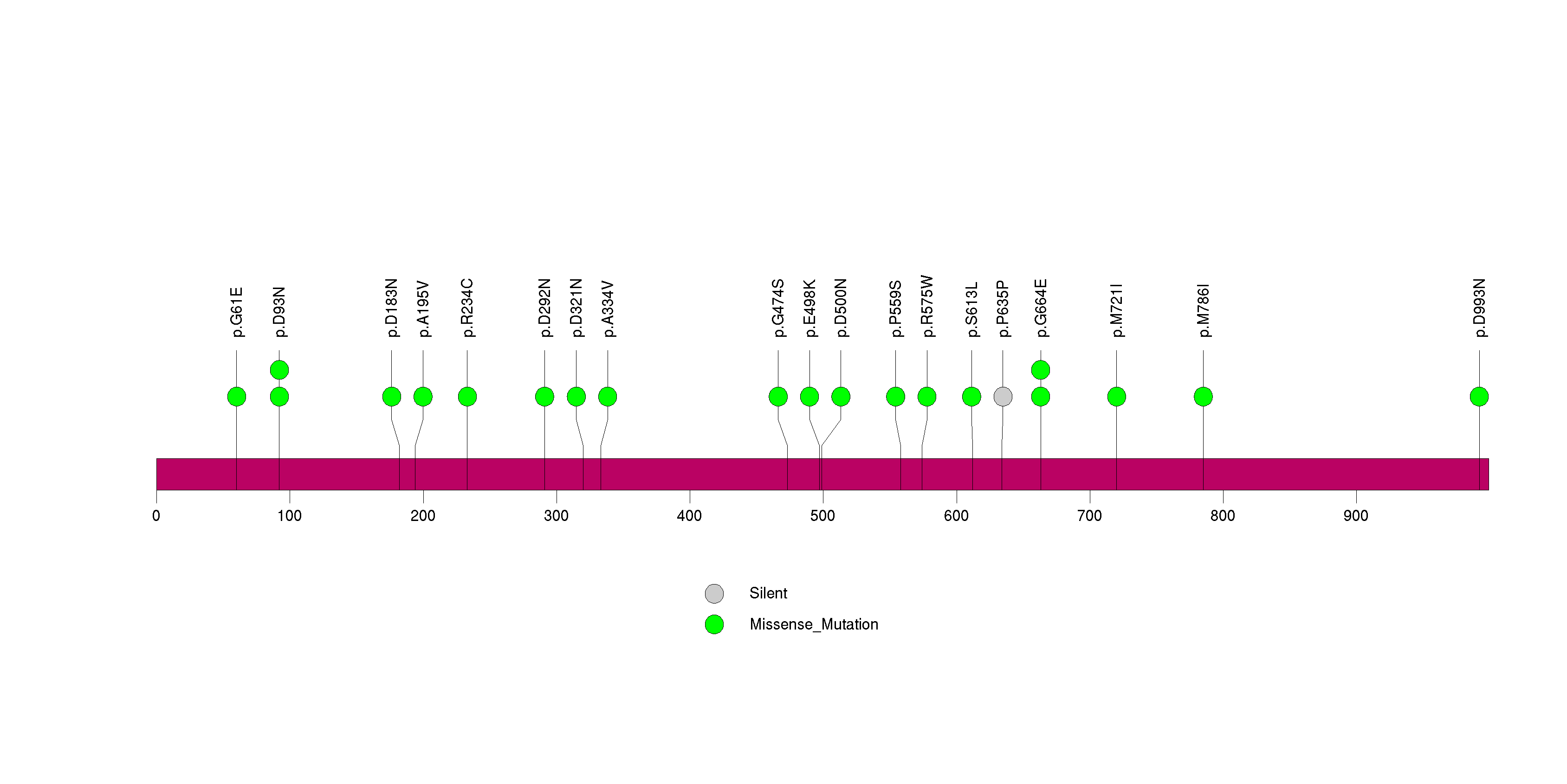
Figure S13. This figure depicts the distribution of mutations and mutation types across the PRAMEF11 significant gene.
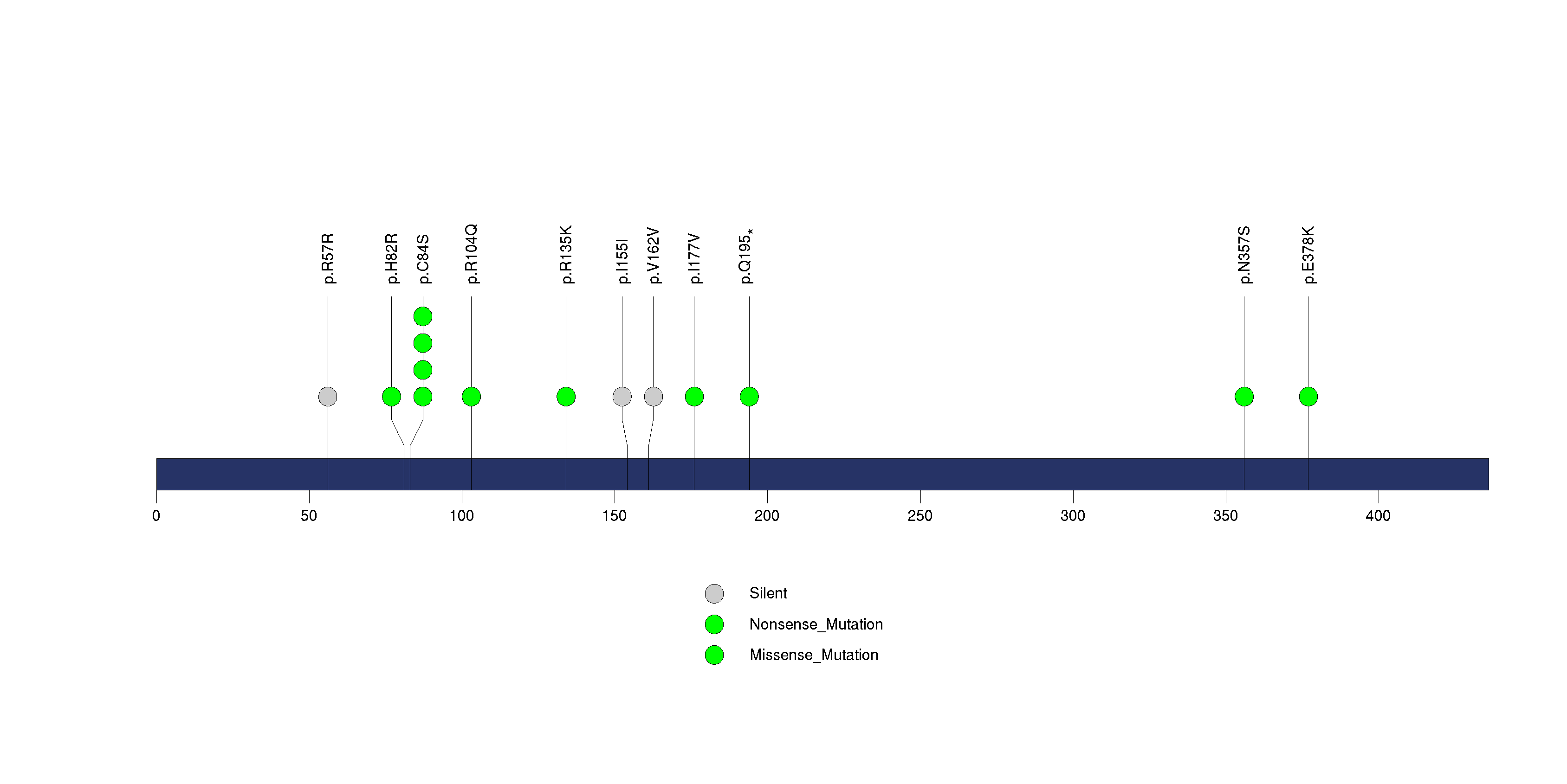
Figure S14. This figure depicts the distribution of mutations and mutation types across the RFX6 significant gene.
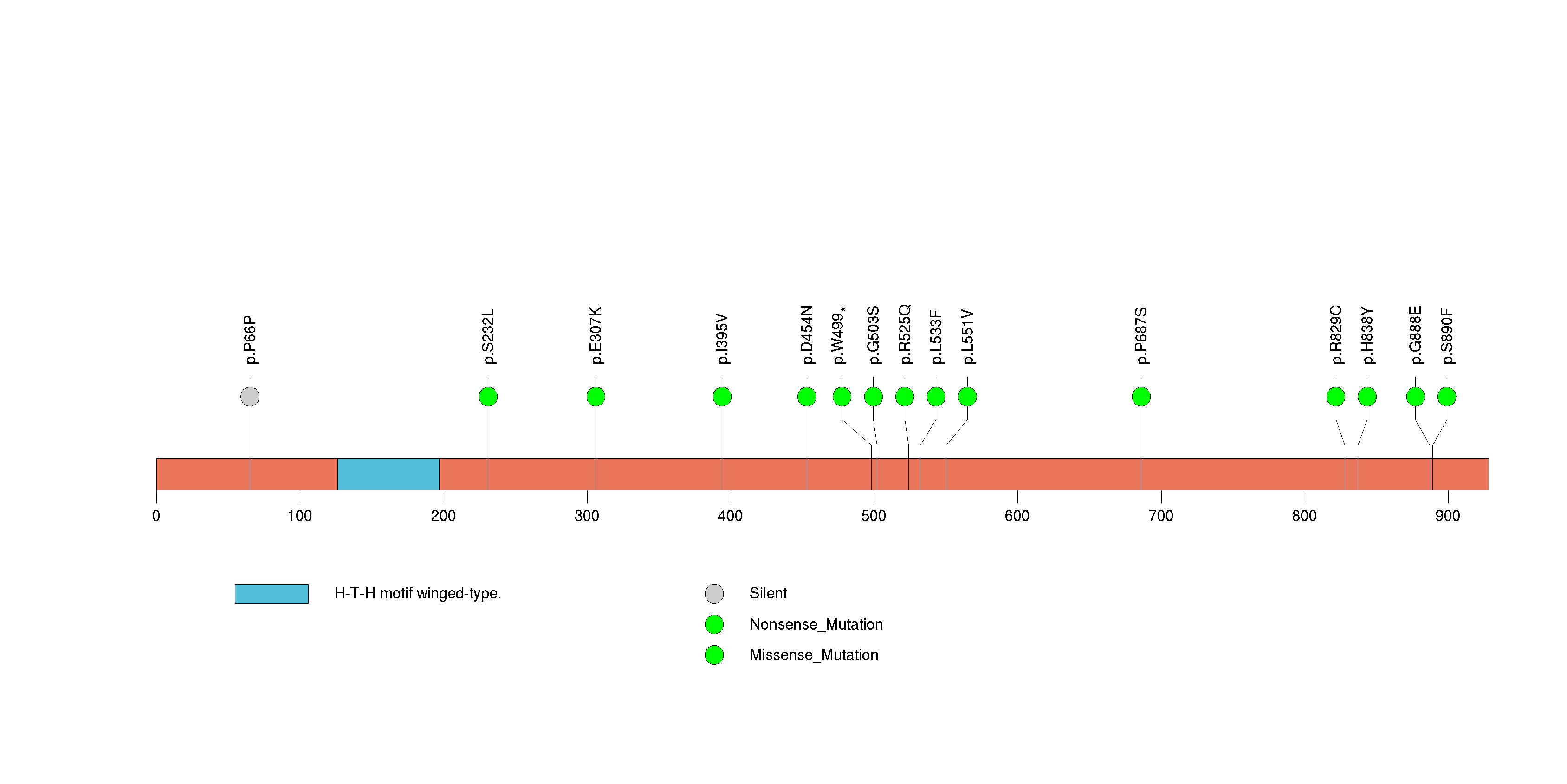
Figure S15. This figure depicts the distribution of mutations and mutation types across the SLC4A10 significant gene.
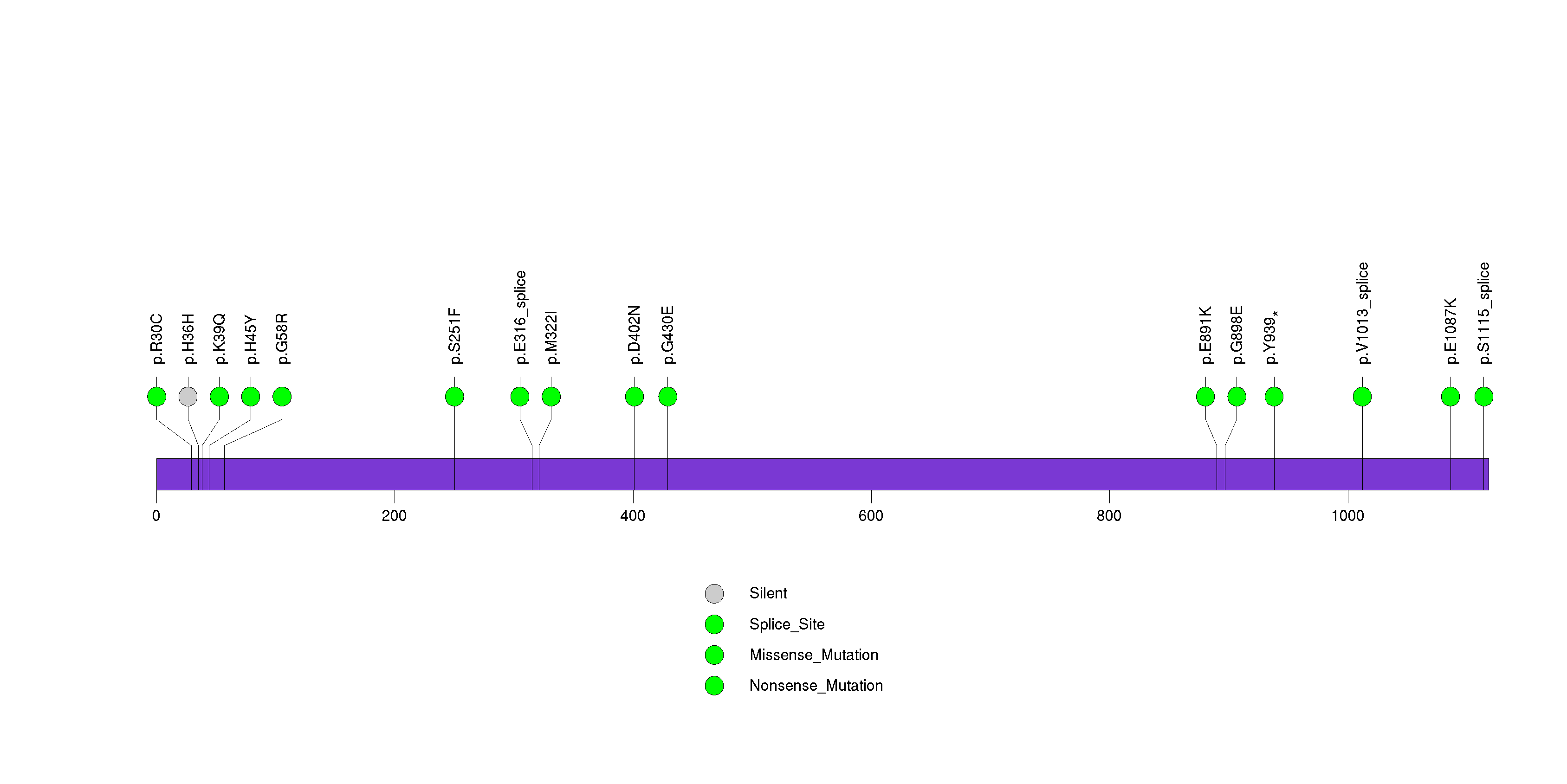
Figure S16. This figure depicts the distribution of mutations and mutation types across the SERPINB4 significant gene.
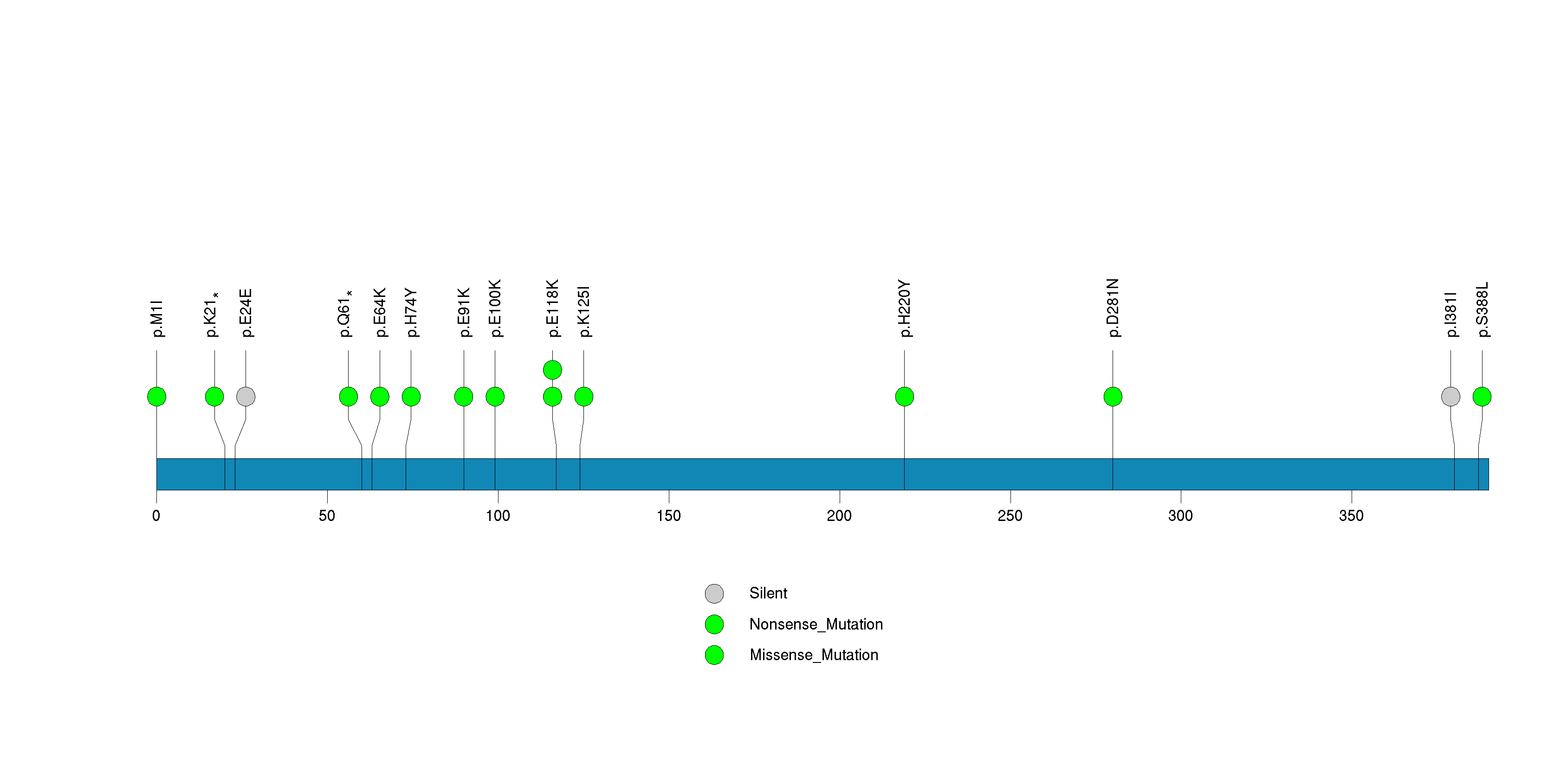
Figure S17. This figure depicts the distribution of mutations and mutation types across the NBPF1 significant gene.
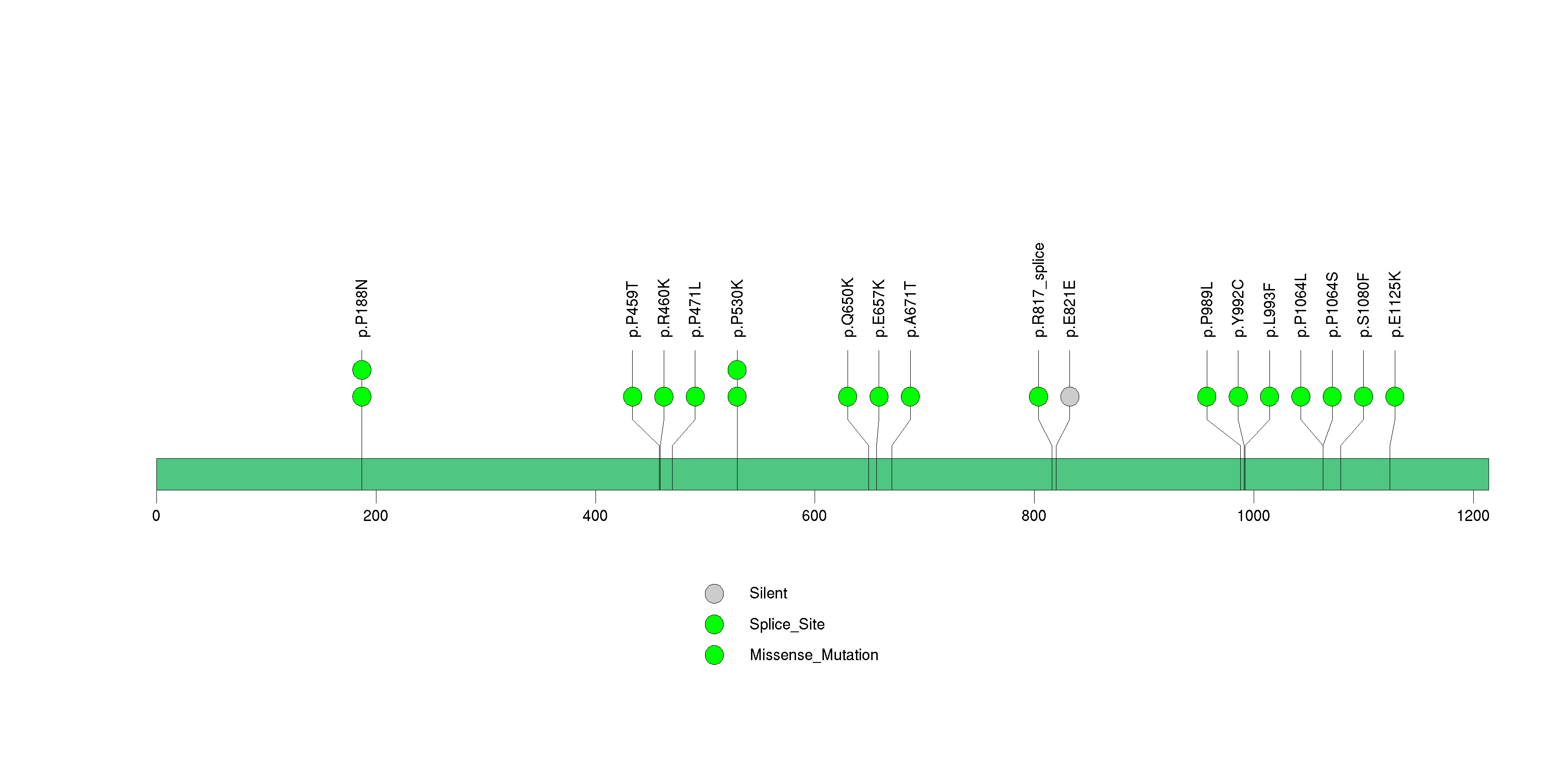
Figure S18. This figure depicts the distribution of mutations and mutation types across the IL32 significant gene.
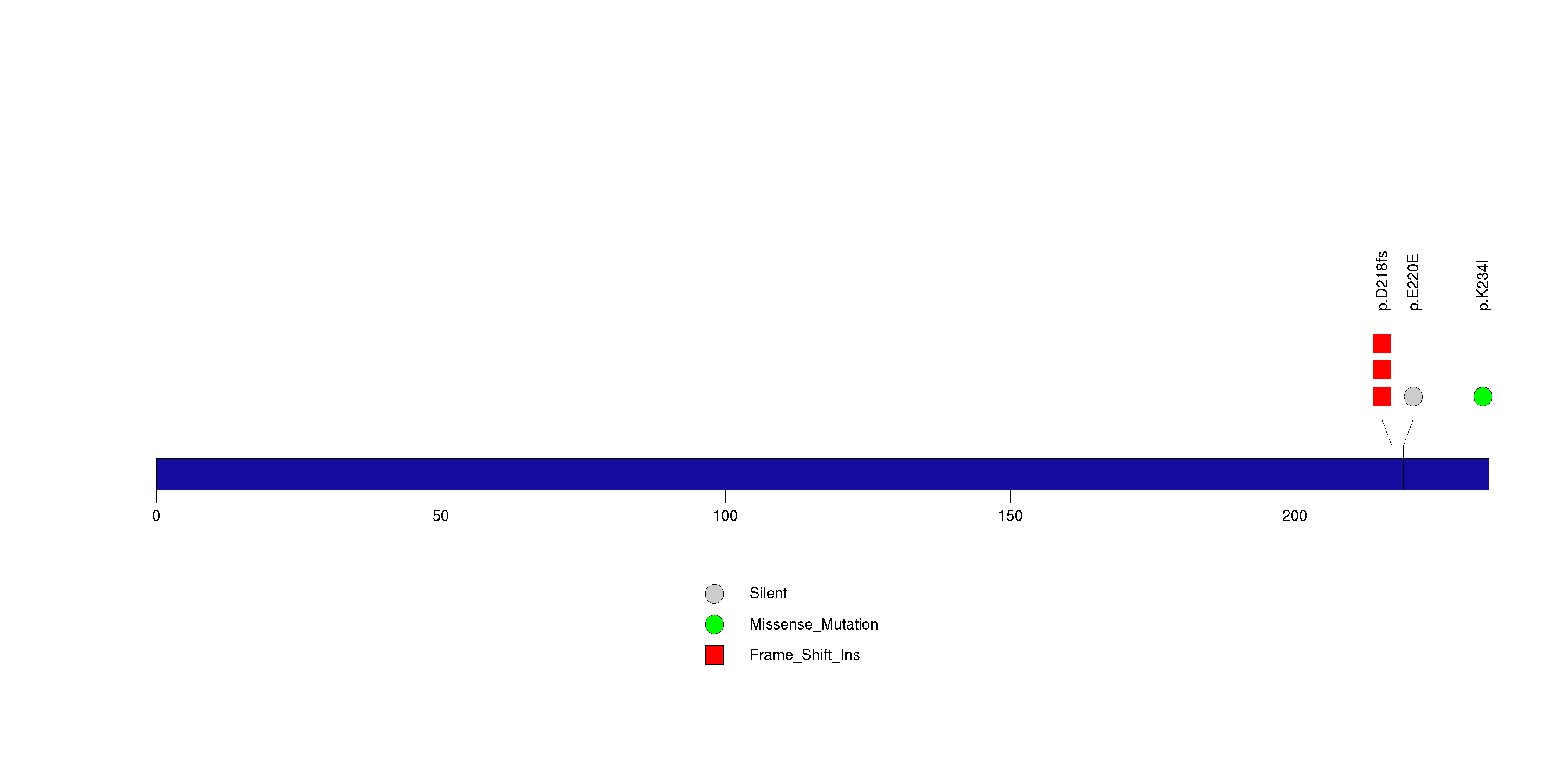
Figure S19. This figure depicts the distribution of mutations and mutation types across the OR4C15 significant gene.
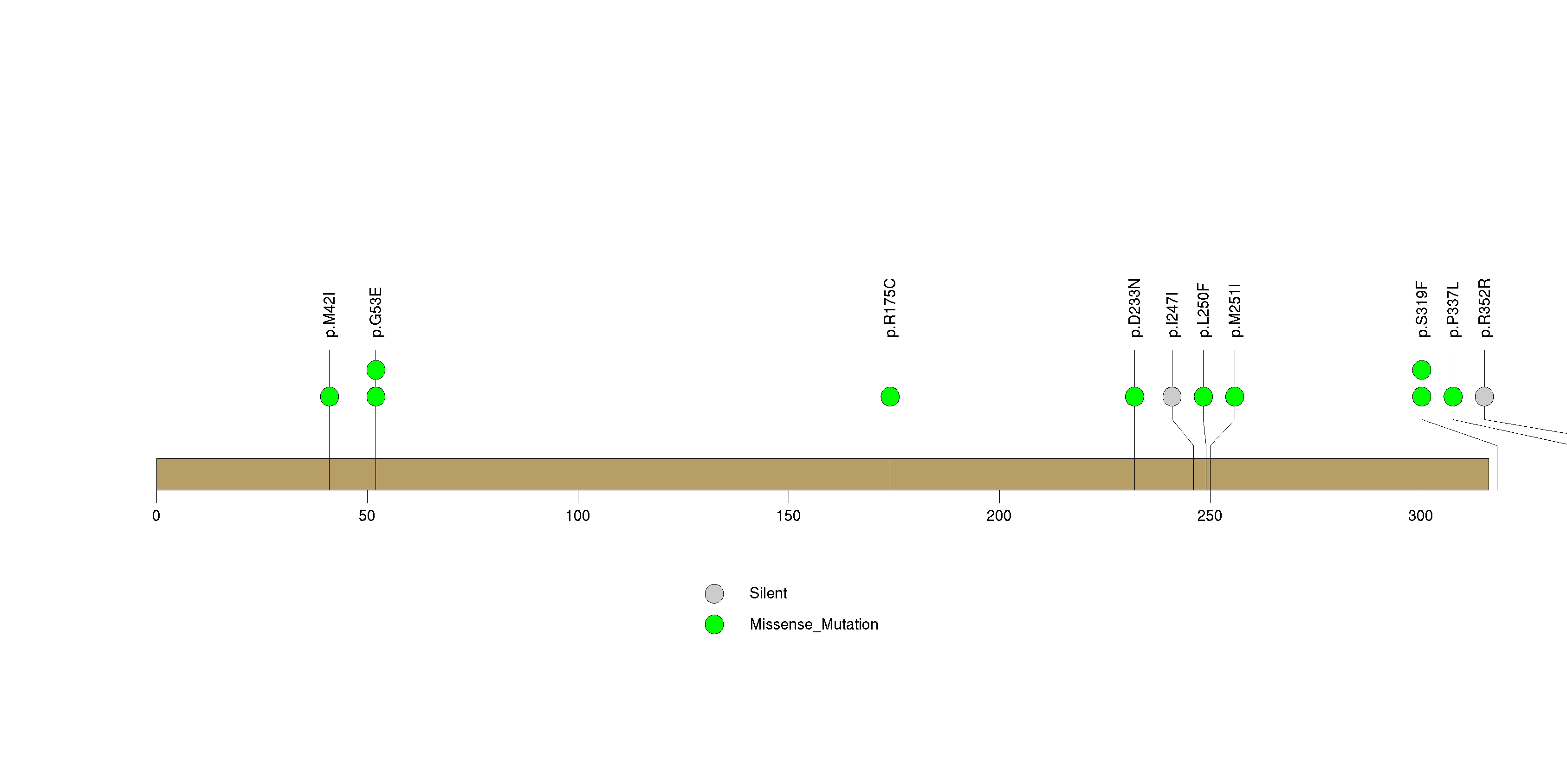
Figure S20. This figure depicts the distribution of mutations and mutation types across the NELL1 significant gene.
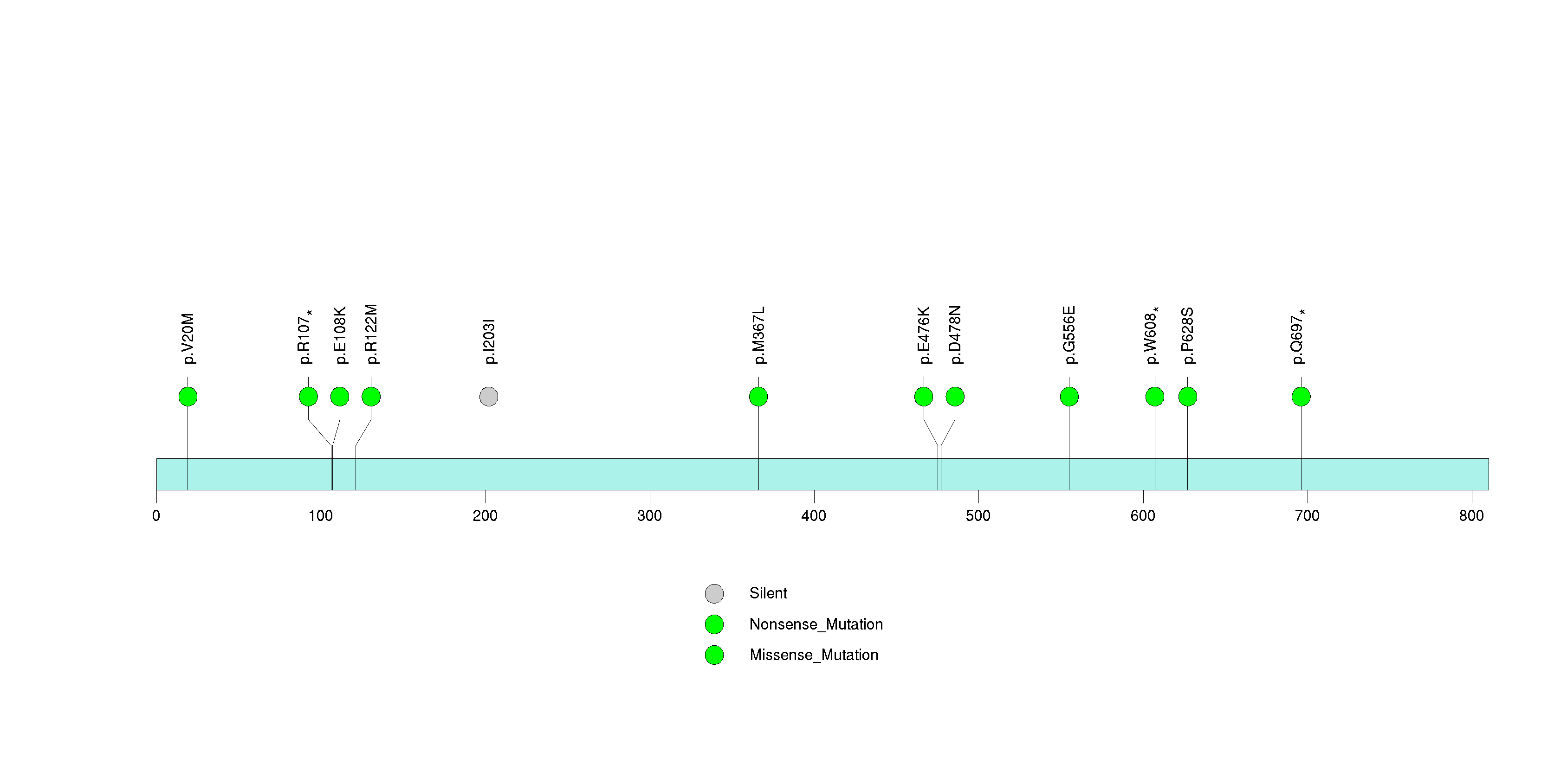
Figure S21. This figure depicts the distribution of mutations and mutation types across the CDR1 significant gene.
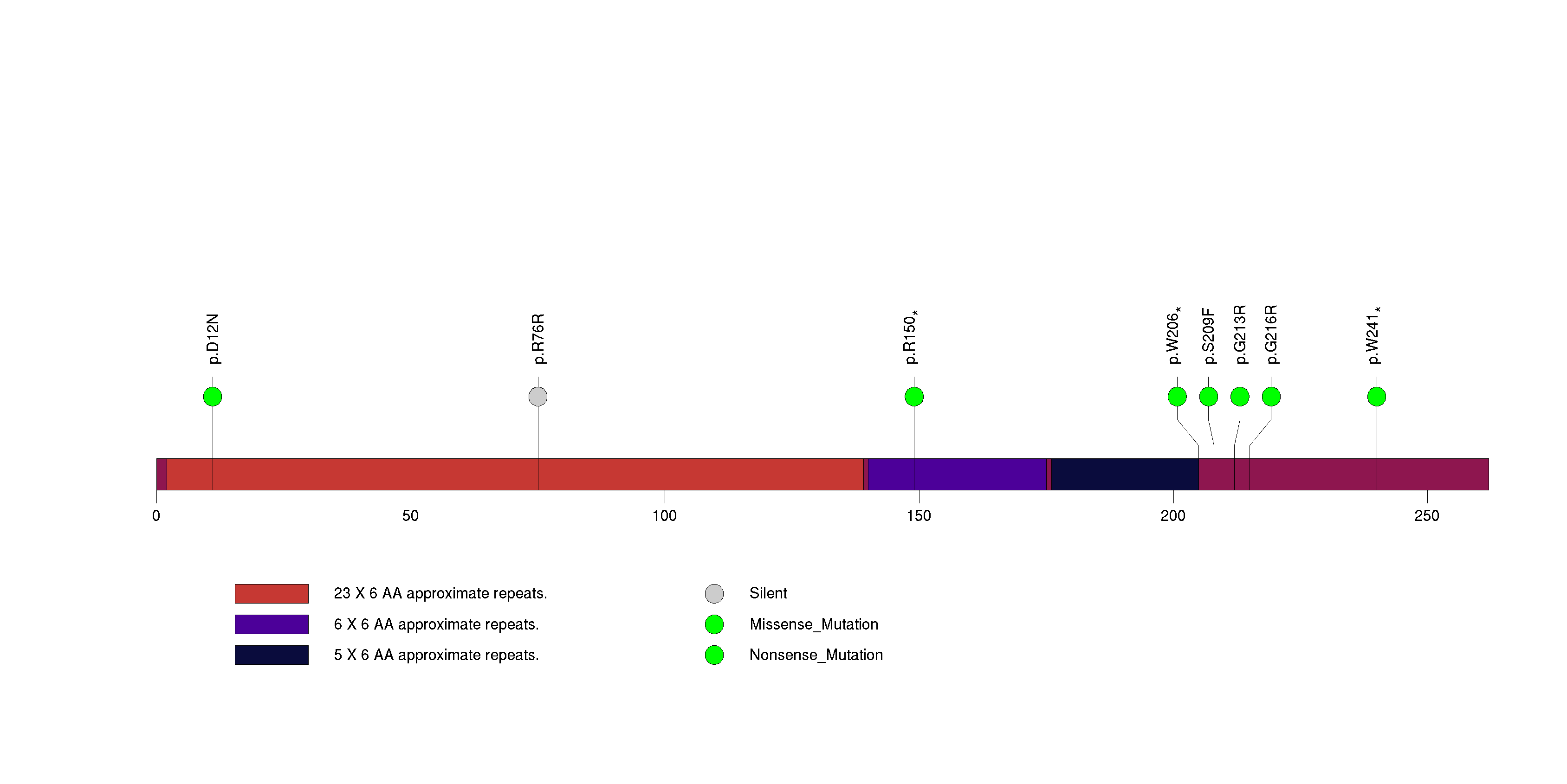
Figure S22. This figure depicts the distribution of mutations and mutation types across the GK2 significant gene.
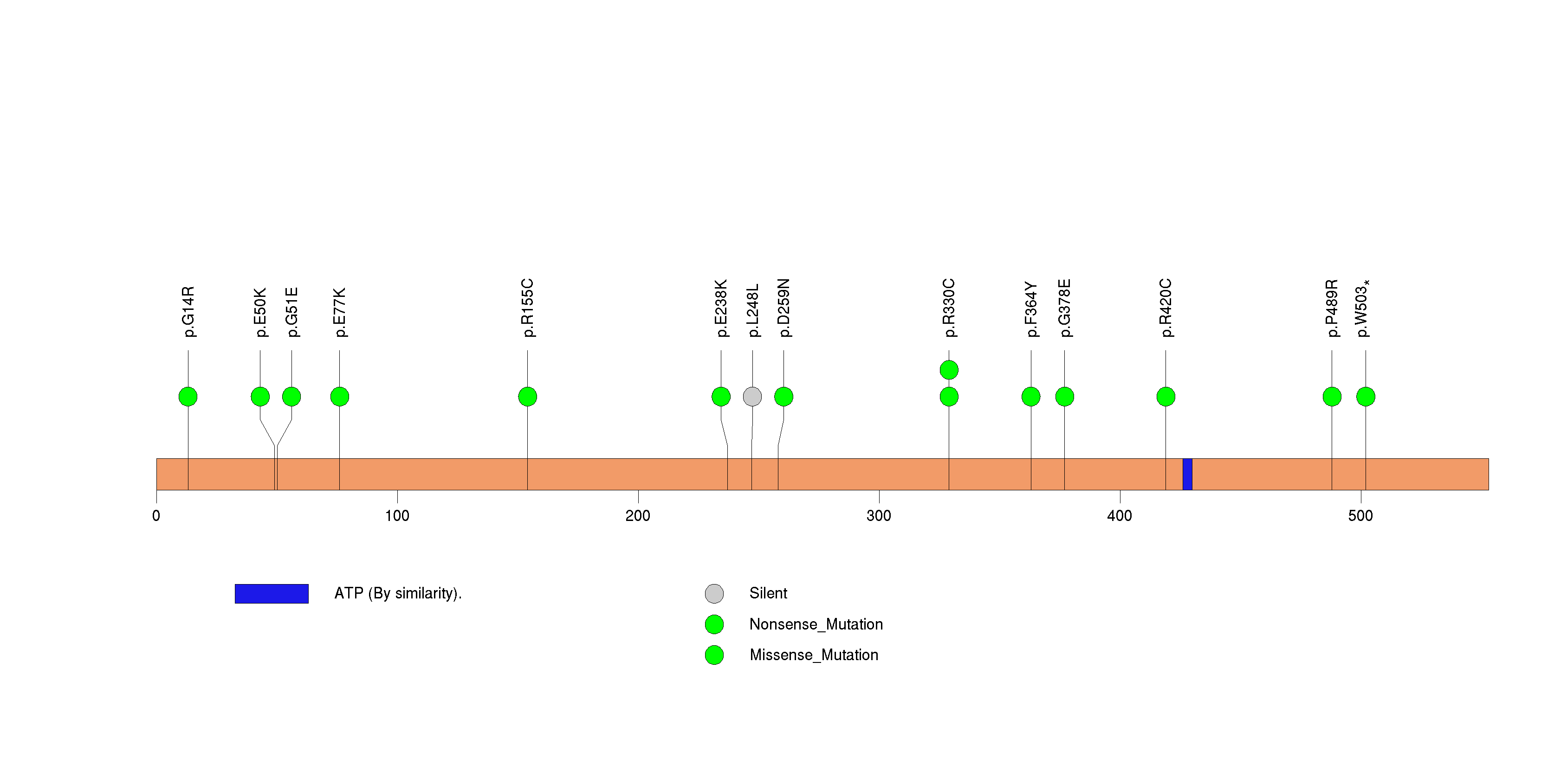
Figure S23. This figure depicts the distribution of mutations and mutation types across the OR5D13 significant gene.
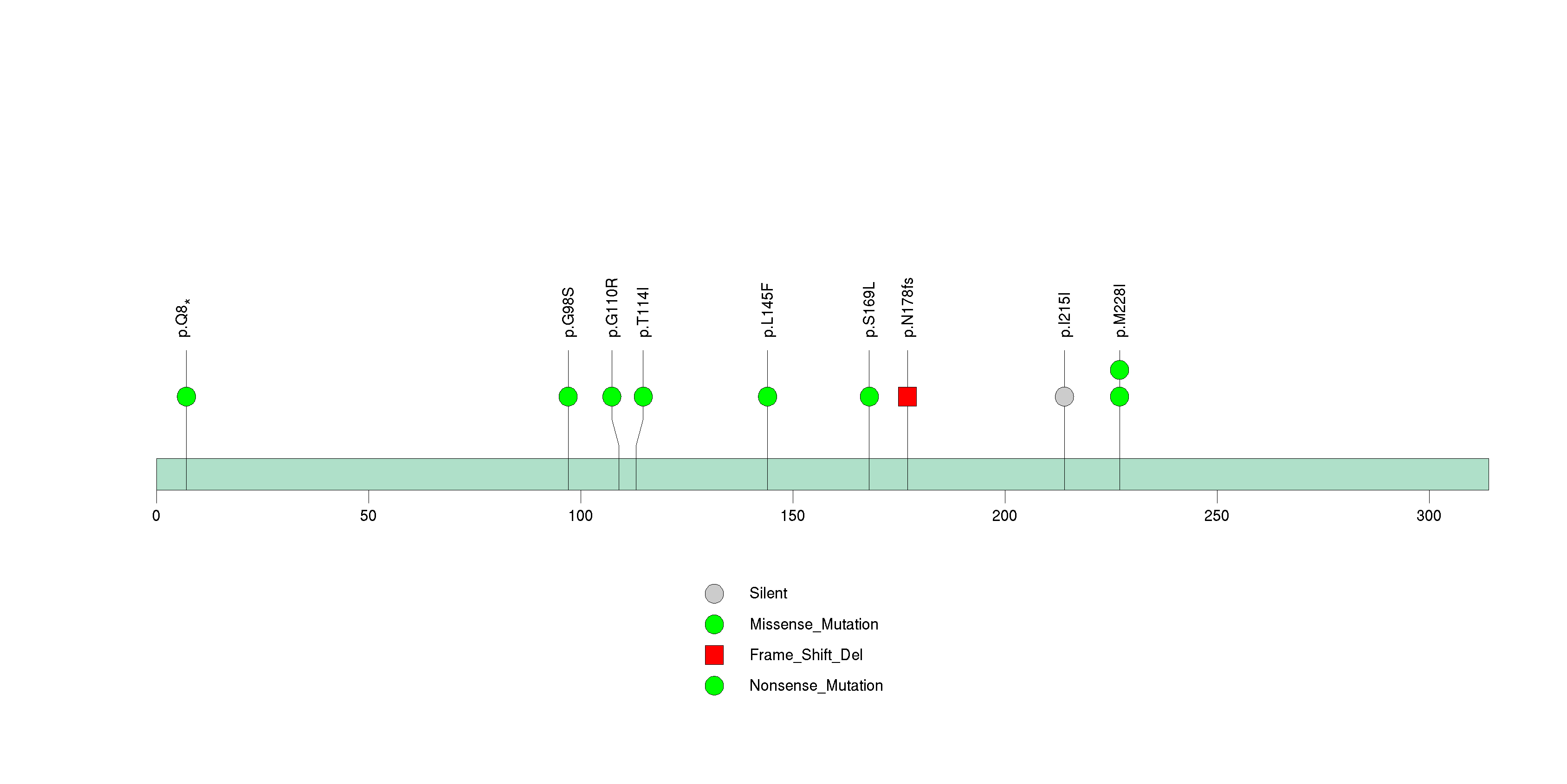
Figure S24. This figure depicts the distribution of mutations and mutation types across the ZNF215 significant gene.
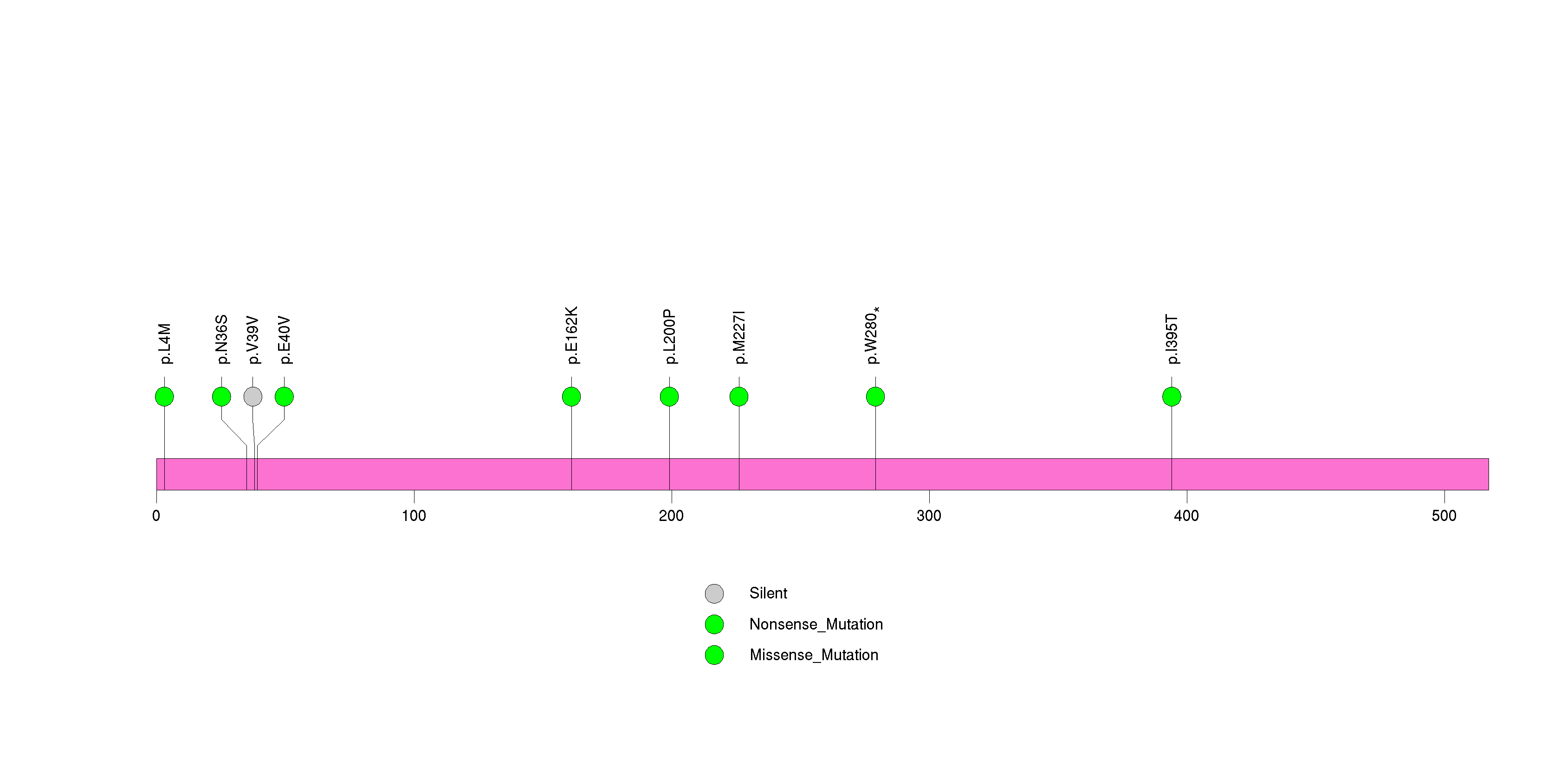
Figure S25. This figure depicts the distribution of mutations and mutation types across the TRHR significant gene.
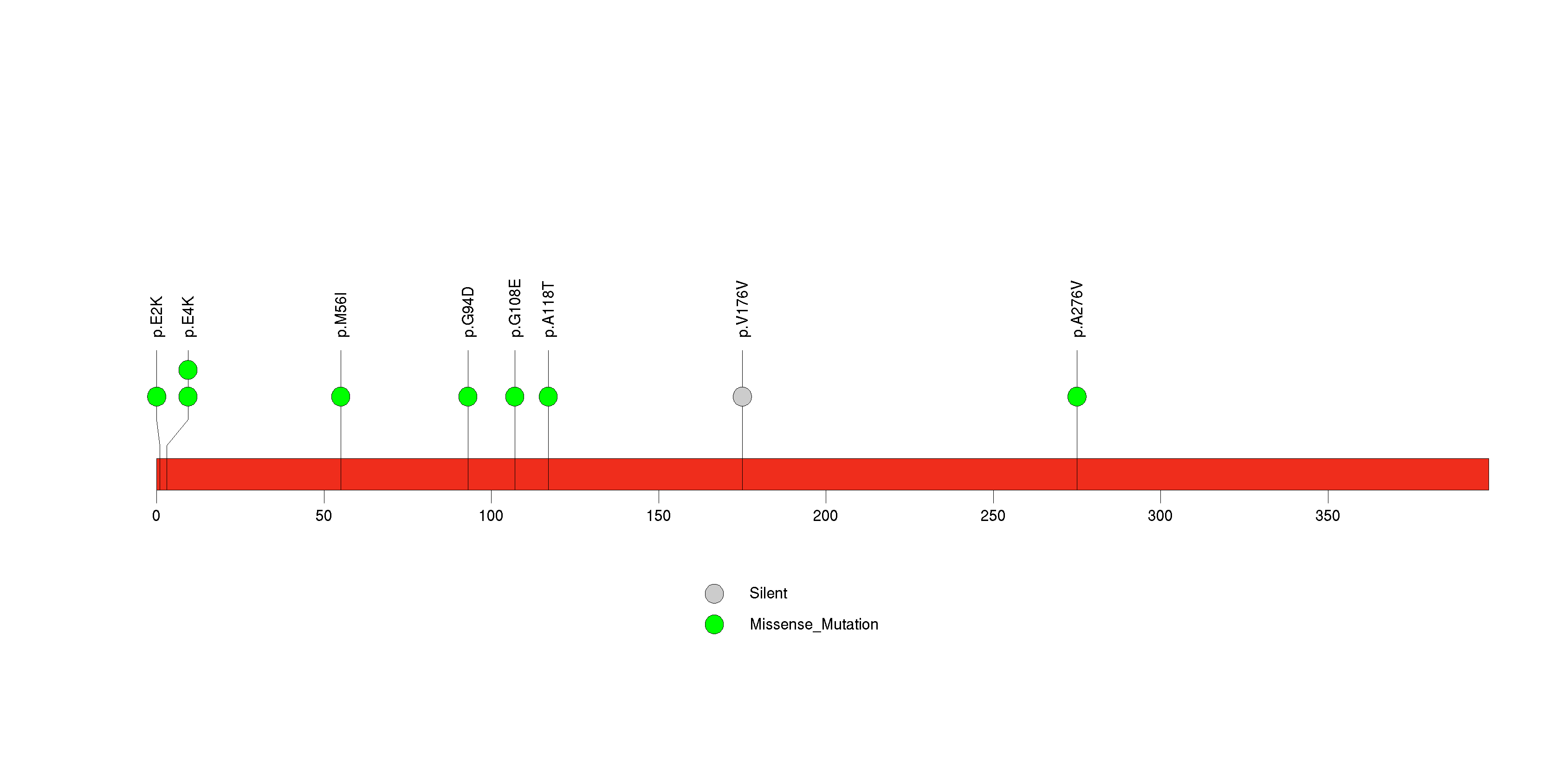
Figure S26. This figure depicts the distribution of mutations and mutation types across the PRSS1 significant gene.
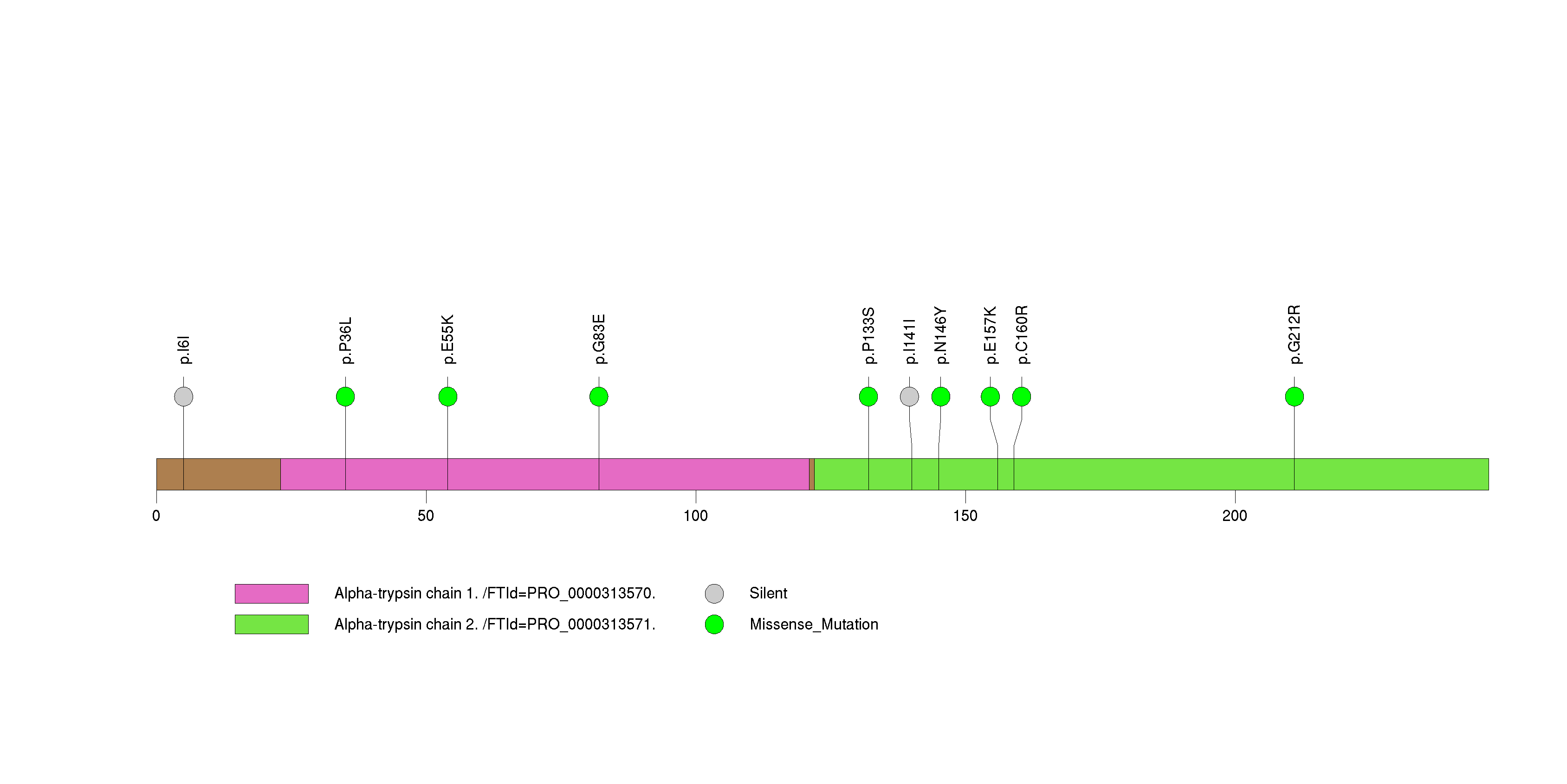
Figure S27. This figure depicts the distribution of mutations and mutation types across the UNC119B significant gene.
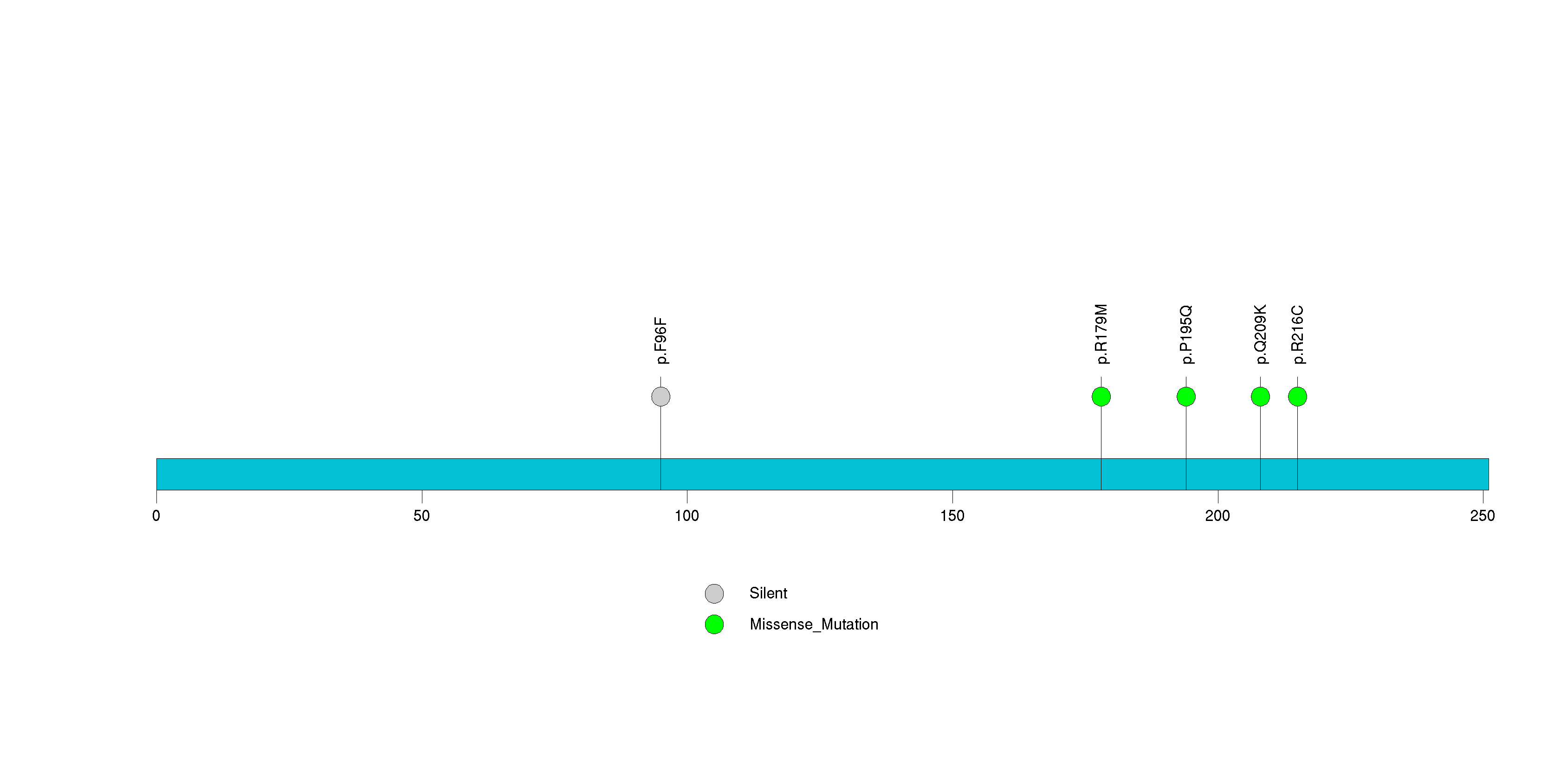
Figure S28. This figure depicts the distribution of mutations and mutation types across the RGS21 significant gene.
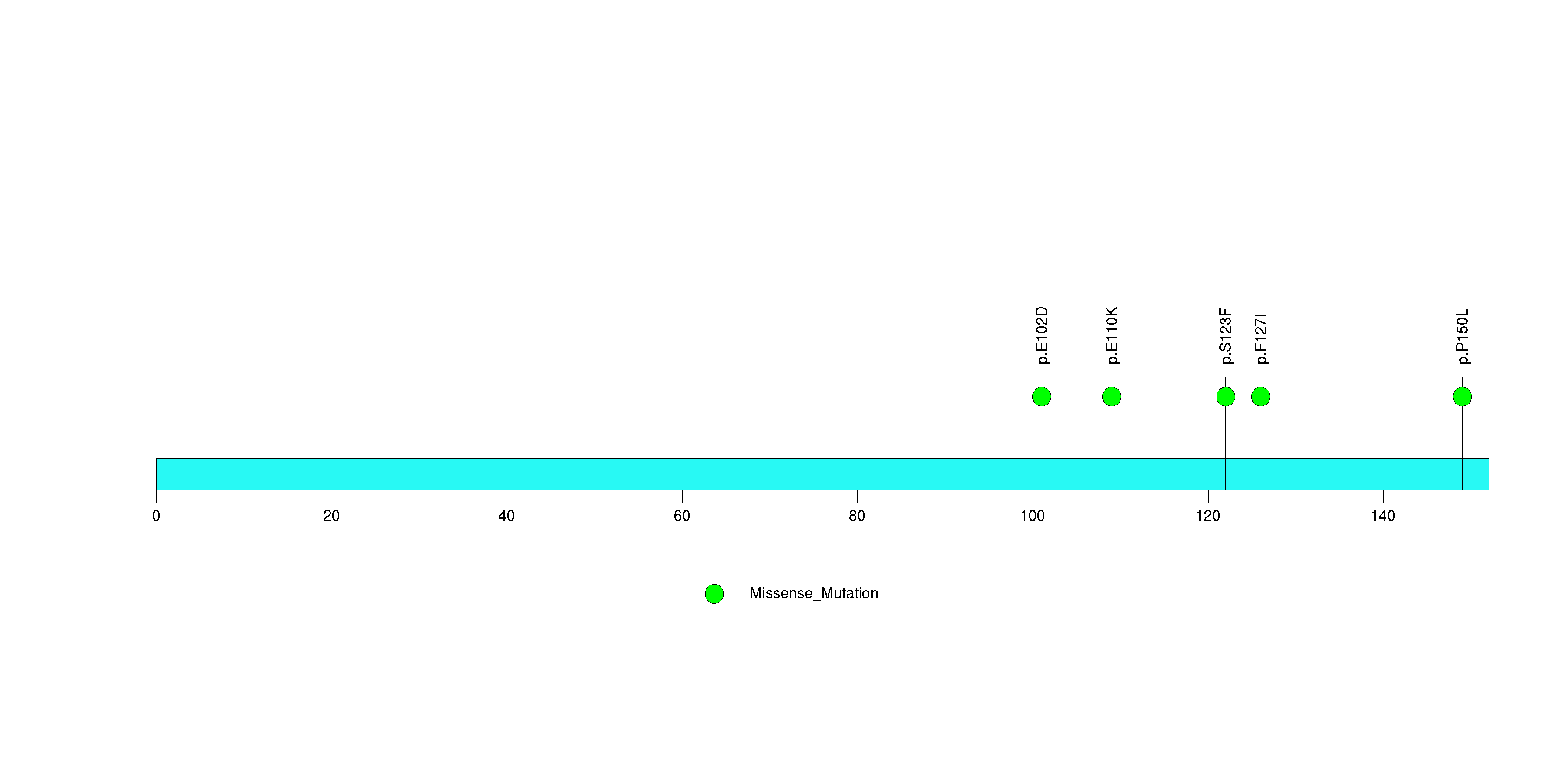
Figure S29. This figure depicts the distribution of mutations and mutation types across the FUT9 significant gene.
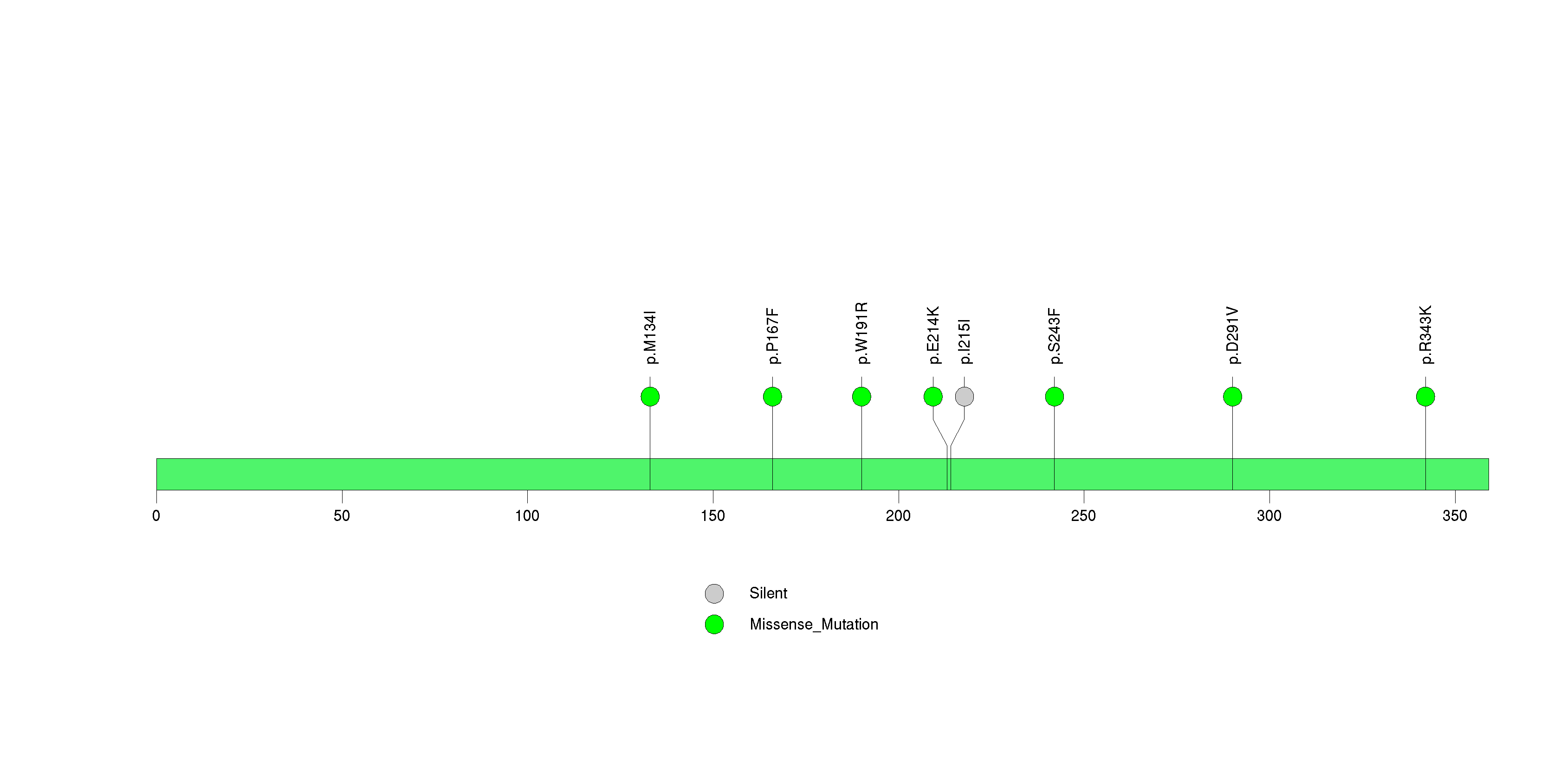
Figure S30. This figure depicts the distribution of mutations and mutation types across the CCDC102B significant gene.
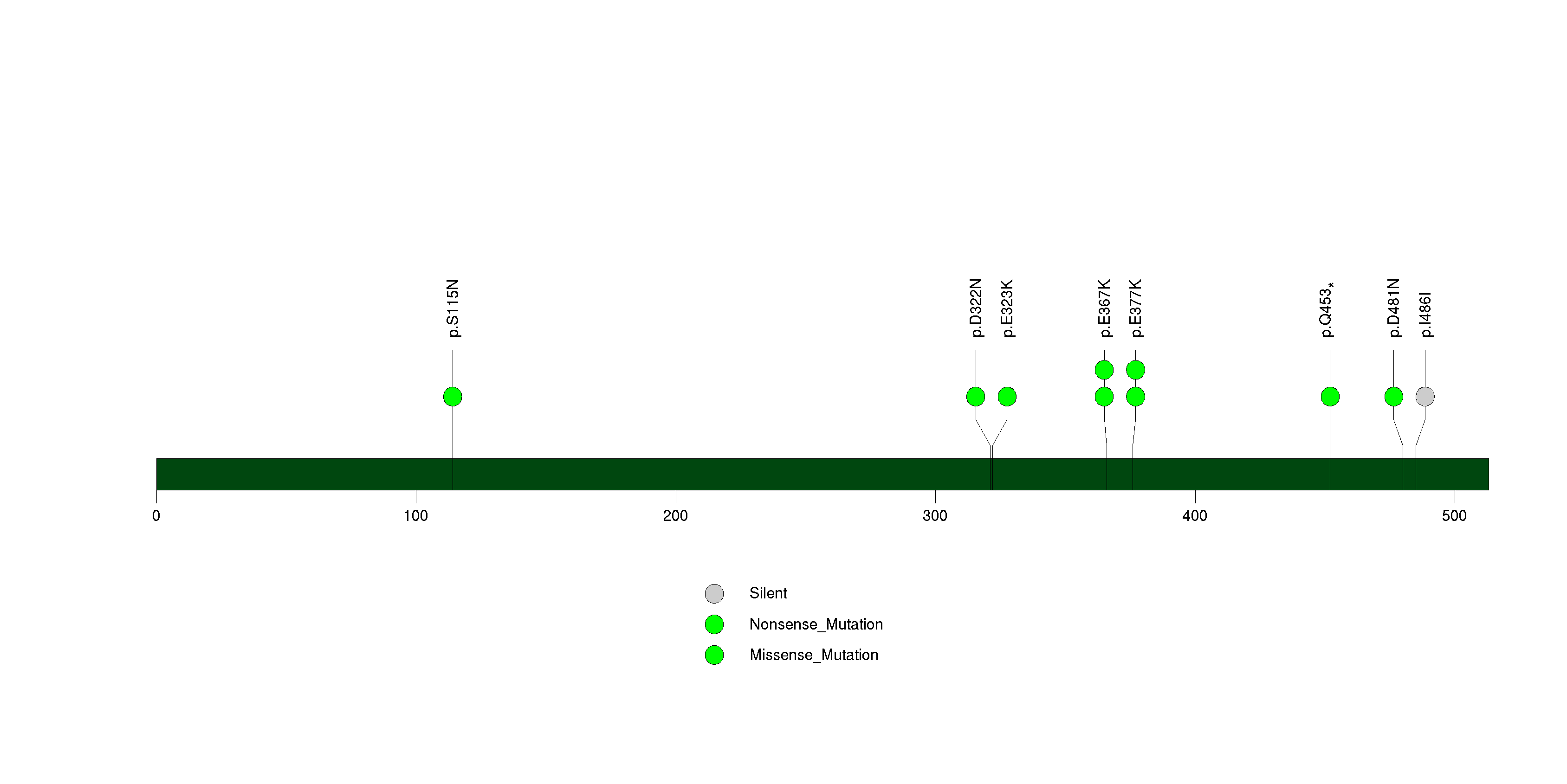
Figure S31. This figure depicts the distribution of mutations and mutation types across the ST6GAL2 significant gene.
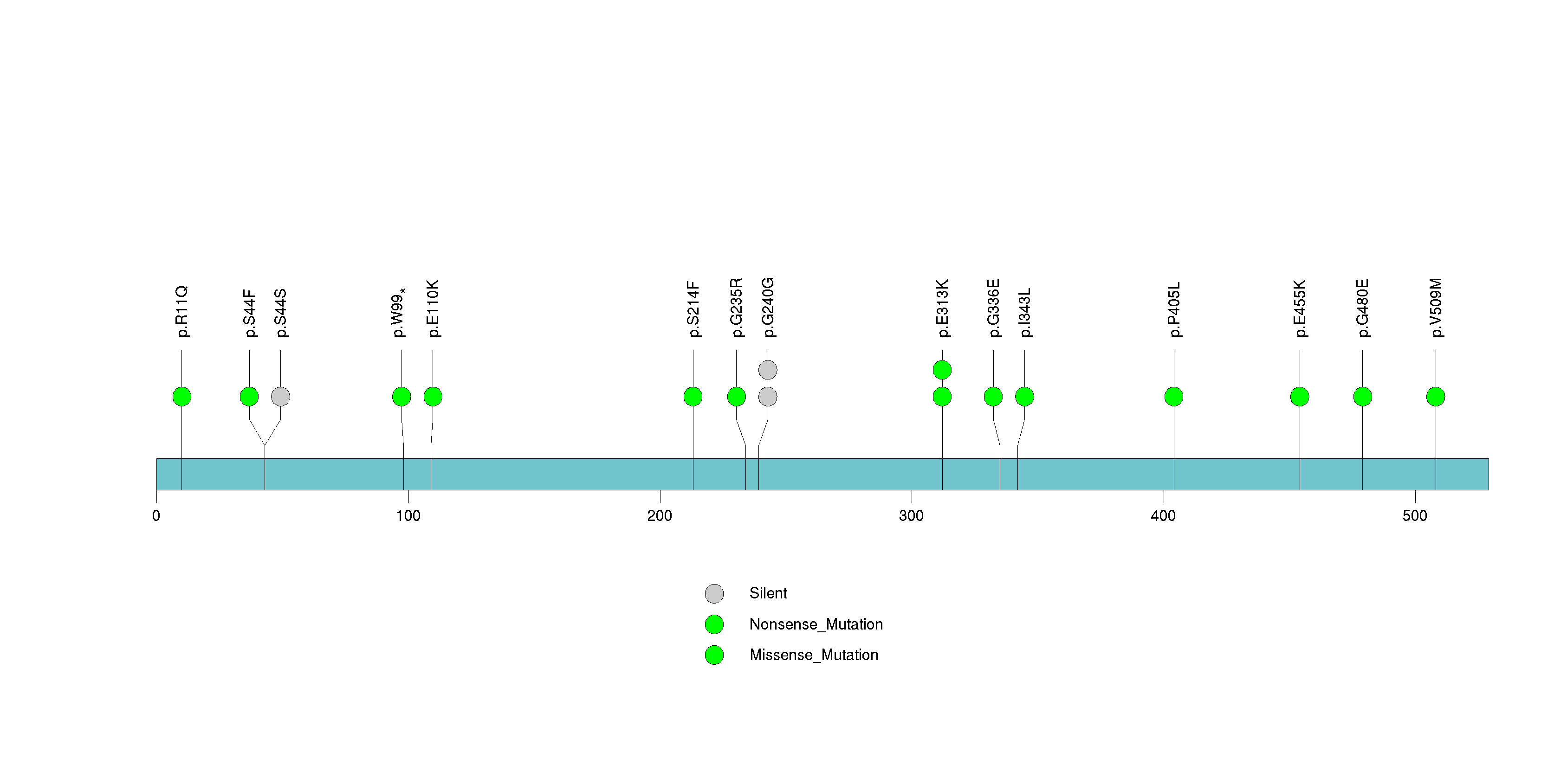
Figure S32. This figure depicts the distribution of mutations and mutation types across the OR11L1 significant gene.
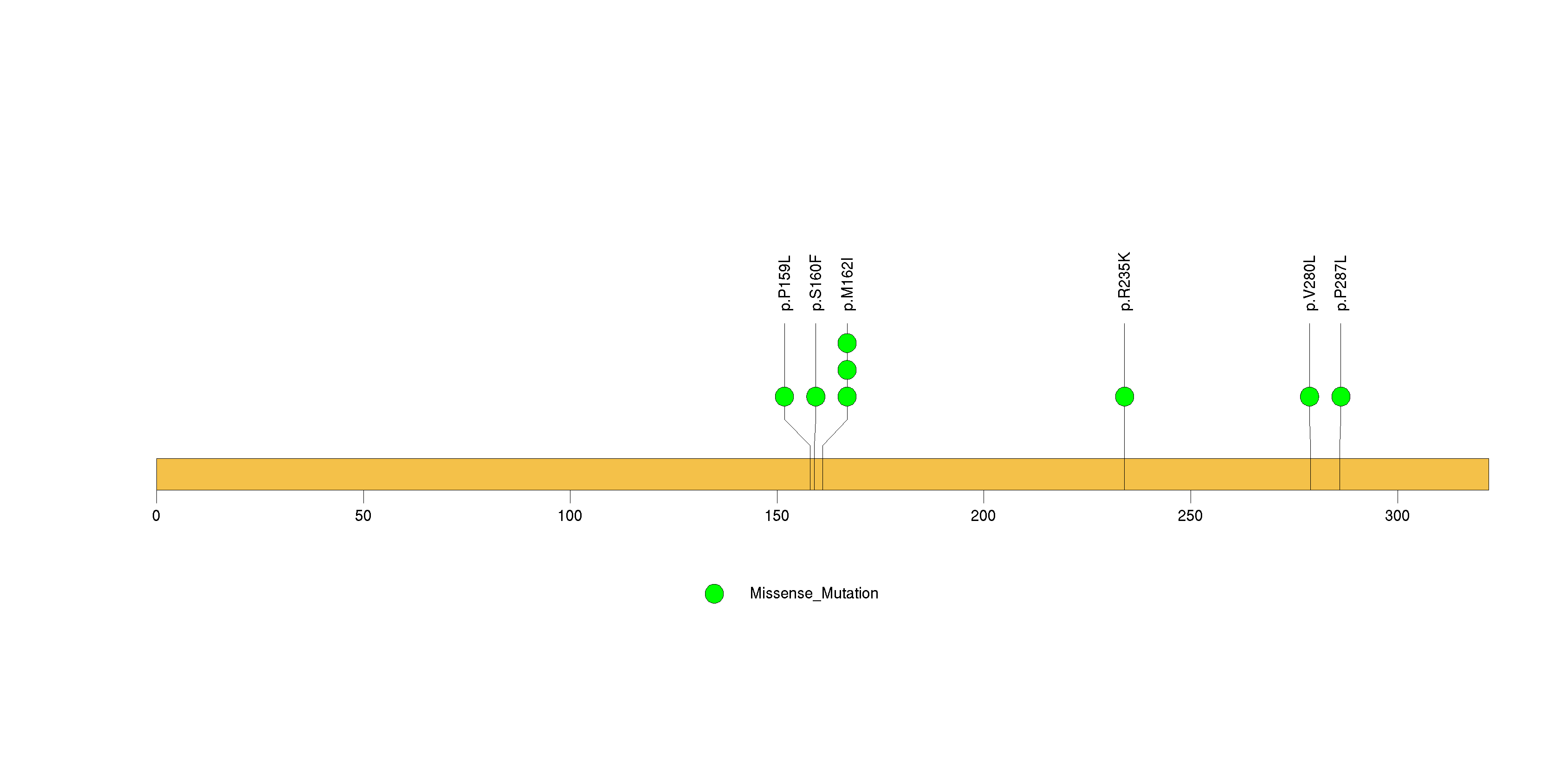
Figure S33. This figure depicts the distribution of mutations and mutation types across the GIMAP4 significant gene.
In this analysis, COSMIC is used as a filter to increase power by restricting the territory of each gene. Cosmic version: v48.
Table 4. Get Full Table Significantly mutated genes (COSMIC territory only). To access the database please go to: COSMIC. Number of significant genes found: 10. Number of genes displayed: 10
| rank | gene | description | n | cos | n_cos | N_cos | cos_ev | p | q |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 99 | 89 | 97 | 8633 | 1393888 | 0 | 0 |
| 2 | CDKN2A | cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | 12 | 332 | 12 | 32204 | 330 | 0 | 0 |
| 3 | TP53 | tumor protein p53 | 11 | 356 | 11 | 34532 | 638 | 1.9e-12 | 2.9e-09 |
| 4 | PTEN | phosphatase and tensin homolog (mutated in multiple advanced cancers 1) | 12 | 767 | 12 | 74399 | 308 | 5.4e-10 | 6.1e-07 |
| 5 | NAP1L2 | nucleosome assembly protein 1-like 2 | 6 | 1 | 2 | 97 | 2 | 7.8e-07 | 0.0007 |
| 6 | CAMTA1 | calmodulin binding transcription activator 1 | 8 | 2 | 2 | 194 | 2 | 3.1e-06 | 0.0018 |
| 7 | CCNA1 | cyclin A1 | 8 | 2 | 2 | 194 | 4 | 3.1e-06 | 0.0018 |
| 8 | STK19 | serine/threonine kinase 19 | 2 | 2 | 2 | 194 | 4 | 3.1e-06 | 0.0018 |
| 9 | IDH1 | isocitrate dehydrogenase 1 (NADP+), soluble | 3 | 5 | 2 | 485 | 2984 | 2e-05 | 0.0098 |
| 10 | DCLK3 | doublecortin-like kinase 3 | 5 | 6 | 2 | 582 | 2 | 0.000028 | 0.013 |
Note:
n - number of (nonsilent) mutations in this gene across the individual set.
cos = number of unique mutated sites in this gene in COSMIC
n_cos = overlap between n and cos.
N_cos = number of individuals times cos.
cos_ev = total evidence: number of reports in COSMIC for mutations seen in this gene.
p = p-value for seeing the observed amount of overlap in this gene)
q = q-value, False Discovery Rate (Benjamini-Hochberg procedure)
Table 5. Get Full Table Genes with Clustered Mutations
| num | gene | desc | n | mindist | nmuts0 | nmuts3 | nmuts12 | npairs0 | npairs3 | npairs12 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1006 | BRAF | v-raf murine sarcoma viral oncogene homolog B1 | 99 | 0 | 3403 | 3403 | 3403 | 3403 | 3403 | 3403 |
| 10684 | TTN | titin | 291 | 0 | 11 | 14 | 30 | 11 | 14 | 30 |
| 6304 | MUC16 | mucin 16, cell surface associated | 227 | 0 | 9 | 25 | 66 | 9 | 25 | 66 |
| 2879 | DNAH5 | dynein, axonemal, heavy chain 5 | 103 | 0 | 8 | 13 | 35 | 8 | 13 | 35 |
| 7436 | PCLO | piccolo (presynaptic cytomatrix protein) | 75 | 0 | 6 | 14 | 20 | 6 | 14 | 20 |
| 7988 | PRAMEF11 | PRAME family member 11 | 11 | 0 | 6 | 10 | 10 | 6 | 10 | 10 |
| 2264 | CNTN5 | contactin 5 | 24 | 0 | 6 | 9 | 12 | 6 | 9 | 12 |
| 729 | ASXL3 | additional sex combs like 3 (Drosophila) | 28 | 0 | 6 | 7 | 11 | 6 | 7 | 11 |
| 8529 | RGS7 | regulator of G-protein signaling 7 | 13 | 0 | 6 | 7 | 7 | 6 | 7 | 7 |
| 2295 | COL21A1 | collagen, type XXI, alpha 1 | 13 | 0 | 6 | 6 | 17 | 6 | 6 | 17 |
Note:
n - number of mutations in this gene in the individual set.
mindist - distance (in aa) between closest pair of mutations in this gene
npairs3 - how many pairs of mutations are within 3 aa of each other.
npairs12 - how many pairs of mutations are within 12 aa of each other.
Table 6. Get Full Table A Ranked List of Significantly Mutated Genesets. (Source: MSigDB GSEA Cannonical Pathway Set).Number of significant genesets found: 2. Number of genesets displayed: 10
| rank | geneset | description | genes | N_genes | mut_tally | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_ns_s | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ST_G_ALPHA_S_PATHWAY | The G-alpha-s protein activates adenylyl cyclases, which catalyze cAMP formation. | ASAH1, BF, BFAR, BRAF, CAMP, CREB1, CREB3, CREB5, EPAC, GAS, GRF2, MAPK1, RAF1, SNX13, SRC, TERF2IP | 12 | BRAF(99), CREB5(2), MAPK1(1), RAF1(3), SNX13(1) | 1602469 | 106 | 97 | 11 | 7 | 6 | 0 | 1 | 98 | 1 | 0 | 0.00014 | 1.9e-15 | 1.2e-12 |
| 2 | ST_ERK1_ERK2_MAPK_PATHWAY | The Erk1 and Erk2 MAP kinase pathways are regulated by Raf, Mos, and Tpl-2. | ARAF1, ATF1, BAD, BRAF, COPEB, CREB1, CREB3, CREB5, DUSP4, DUSP6, DUSP9, EEF2K, EIF4E, GRB2, HTATIP, MAP2K1, MAP2K2, MAP3K8, MAPK1, MAPK3, MKNK1, MKNK2, MOS, NFKB1, RAP1A, RPS6KA1, RPS6KA2, RPS6KA3, SHC1, SOS1, SOS2, TRAF3 | 29 | ATF1(3), BRAF(99), CREB5(2), DUSP6(1), GRB2(1), MAP2K1(3), MAP2K2(2), MAPK1(1), MKNK1(2), MOS(1), NFKB1(1), RPS6KA1(1), RPS6KA2(5), RPS6KA3(1), SOS1(3), SOS2(4), TRAF3(2) | 4188759 | 132 | 97 | 35 | 21 | 22 | 1 | 2 | 104 | 3 | 0 | 0.0072 | 3.7e-14 | 1.1e-11 |
| 3 | SA_G1_AND_S_PHASES | Cdk2, 4, and 6 bind cyclin D in G1, while cdk2/cyclin E promotes the G1/S transition. | ARF1, ARF3, CCND1, CDK2, CDK4, CDKN1A, CDKN1B, CDKN2A, CFL1, E2F1, E2F2, MDM2, NXT1, PRB1, TP53 | 15 | CDK4(4), CDKN1A(1), CDKN2A(12), CFL1(1), E2F2(1), MDM2(2), PRB1(7), TP53(11) | 1208404 | 39 | 27 | 35 | 5 | 13 | 1 | 2 | 10 | 13 | 0 | 0.0074 | 0.0013 | 0.27 |
| 4 | SA_REG_CASCADE_OF_CYCLIN_EXPR | Expression of cyclins regulates progression through the cell cycle by activating cyclin-dependent kinases. | CCNA1, CCNA2, CCND1, CCNE1, CCNE2, CDK2, CDK4, CDKN1B, CDKN2A, E2F1, E2F2, E2F4, PRB1 | 13 | CCNA1(8), CCNE1(1), CCNE2(5), CDK4(4), CDKN2A(12), E2F2(1), PRB1(7) | 1286870 | 38 | 28 | 34 | 5 | 19 | 0 | 1 | 10 | 8 | 0 | 0.0088 | 0.0028 | 0.43 |
| 5 | HSA04150_MTOR_SIGNALING_PATHWAY | Genes involved in mTOR signaling pathway | AKT1, AKT2, AKT3, BRAF, CAB39, DDIT4, EIF4B, EIF4EBP1, FIGF, FRAP1, GBL, HIF1A, IGF1, INS, KIAA1303, LYK5, MAPK1, MAPK3, PDPK1, PGF, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PIK3R1, PIK3R2, PIK3R3, PIK3R5, PRKAA1, PRKAA2, RHEB, RICTOR, RPS6, RPS6KA1, RPS6KA2, RPS6KA3, RPS6KA6, RPS6KB1, RPS6KB2, STK11, TSC1, TSC2, ULK1, ULK2, ULK3, VEGFA, VEGFB, VEGFC | 44 | AKT1(3), AKT2(2), AKT3(1), BRAF(99), FIGF(2), HIF1A(3), IGF1(5), MAPK1(1), PDPK1(1), PIK3CA(3), PIK3CB(9), PIK3CD(3), PIK3CG(8), PIK3R1(3), PIK3R2(3), PIK3R5(7), PRKAA2(6), RICTOR(2), RPS6KA1(1), RPS6KA2(5), RPS6KA3(1), RPS6KA6(7), STK11(2), TSC1(1), TSC2(3), ULK1(1), ULK2(3), ULK3(1), VEGFA(2), VEGFB(1), VEGFC(6) | 7494768 | 195 | 97 | 99 | 34 | 51 | 3 | 4 | 125 | 11 | 1 | 0.000081 | 0.021 | 1 |
| 6 | ST_DIFFERENTIATION_PATHWAY_IN_PC12_CELLS | Rat-derived PC12 cells respond to nerve growth factor (NGF) and PACAP to differentiate into neuronal cells. | AKT1, ASAH1, ATF1, BRAF, CAMP, CREB1, CREB3, CREB5, CREBBP, CRKL, DAG1, EGR1, EGR2, EGR3, EGR4, ELK1, FRS2, GAS, GNAQ, GRF2, JUN, MAP1B, MAP2K4, MAP2K7, MAPK1, MAPK10, MAPK3, MAPK8, MAPK8IP1, MAPK8IP2, MAPK8IP3, MAPK9, NTRK1, OPN1LW, PACAP, PIK3C2G, PIK3CA, PIK3CD, PIK3R1, PTPN11, RPS6KA3, SH2B, SHC1, SRC, TERF2IP, TH, TUBA3 | 42 | AKT1(3), ATF1(3), BRAF(99), CREB5(2), CREBBP(4), CRKL(1), DAG1(1), EGR2(2), EGR4(1), ELK1(1), FRS2(1), GNAQ(1), JUN(1), MAP1B(5), MAP2K4(1), MAP2K7(1), MAPK1(1), MAPK10(2), MAPK8IP1(1), MAPK8IP2(1), MAPK9(1), NTRK1(5), OPN1LW(1), PIK3C2G(10), PIK3CA(3), PIK3CD(3), PIK3R1(3), PTPN11(2), RPS6KA3(1), TH(4) | 7267574 | 165 | 97 | 70 | 30 | 40 | 5 | 2 | 113 | 4 | 1 | 0.00077 | 0.021 | 1 |
| 7 | IL5PATHWAY | Pro-inflammatory IL-5 is secretes by activated T cells, eosinophils, and mast cells, and stimulates the proliferation and activation of eosinophils in bone marrow. | CCL11, CCR3, CD4, HLA-DRA, HLA-DRB1, IL1B, IL4, IL5, IL5RA, IL6 | 10 | CCL11(1), CCR3(5), CD4(5), HLA-DRA(3), HLA-DRB1(1), IL1B(2), IL5(1), IL5RA(3) | 761782 | 21 | 19 | 20 | 4 | 15 | 1 | 0 | 4 | 1 | 0 | 0.01 | 0.03 | 1 |
| 8 | STEROID_BIOSYNTHESIS | CYP17A1, F13B, HSD17B1, HSD17B2, HSD17B3, HSD17B4, HSD17B7, HSD3B1, HSD3B2 | 9 | CYP17A1(2), F13B(7), HSD17B2(3), HSD17B4(4), HSD3B1(3), HSD3B2(3) | 1145924 | 22 | 20 | 21 | 3 | 16 | 0 | 2 | 2 | 1 | 1 | 0.007 | 0.034 | 1 | |
| 9 | SLRPPATHWAY | Small leucine-rich proteoglycans (SLRPs) interact with and reorganize collagen fibers in the extracellular matrix. | BGN, DCN, DSPG3, FMOD, KERA, LUM | 5 | BGN(3), DCN(7), KERA(2), LUM(4) | 506483 | 16 | 13 | 16 | 4 | 11 | 2 | 0 | 0 | 3 | 0 | 0.032 | 0.06 | 1 |
| 10 | BBCELLPATHWAY | Fas ligand expression by T cells induces apoptosis in Fas-expressing, inactive B cells. | CD28, CD4, HLA-DRA, HLA-DRB1, TNFRSF5, TNFRSF6, TNFSF5, TNFSF6 | 4 | CD4(5), HLA-DRA(3), HLA-DRB1(1) | 334543 | 9 | 8 | 9 | 0 | 5 | 1 | 0 | 3 | 0 | 0 | 0.019 | 0.077 | 1 |
Table 7. Get Full Table A Ranked List of Significantly Mutated Genesets (Excluding Significantly Mutated Genes). Number of significant genesets found: 0. Number of genesets displayed: 10
| rank | geneset | description | genes | N_genes | mut_tally | N | n | npat | nsite | nsil | n1 | n2 | n3 | n4 | n5 | n6 | p_ns_s | p | q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IL5PATHWAY | Pro-inflammatory IL-5 is secretes by activated T cells, eosinophils, and mast cells, and stimulates the proliferation and activation of eosinophils in bone marrow. | CCL11, CCR3, CD4, HLA-DRA, HLA-DRB1, IL1B, IL4, IL5, IL5RA, IL6 | 10 | CCL11(1), CCR3(5), CD4(5), HLA-DRA(3), HLA-DRB1(1), IL1B(2), IL5(1), IL5RA(3) | 761782 | 21 | 19 | 20 | 4 | 15 | 1 | 0 | 4 | 1 | 0 | 0.01 | 0.03 | 1 |
| 2 | STEROID_BIOSYNTHESIS | CYP17A1, F13B, HSD17B1, HSD17B2, HSD17B3, HSD17B4, HSD17B7, HSD3B1, HSD3B2 | 9 | CYP17A1(2), F13B(7), HSD17B2(3), HSD17B4(4), HSD3B1(3), HSD3B2(3) | 1145924 | 22 | 20 | 21 | 3 | 16 | 0 | 2 | 2 | 1 | 1 | 0.007 | 0.034 | 1 | |
| 3 | SLRPPATHWAY | Small leucine-rich proteoglycans (SLRPs) interact with and reorganize collagen fibers in the extracellular matrix. | BGN, DCN, DSPG3, FMOD, KERA, LUM | 5 | BGN(3), DCN(7), KERA(2), LUM(4) | 506483 | 16 | 13 | 16 | 4 | 11 | 2 | 0 | 0 | 3 | 0 | 0.032 | 0.06 | 1 |
| 4 | BBCELLPATHWAY | Fas ligand expression by T cells induces apoptosis in Fas-expressing, inactive B cells. | CD28, CD4, HLA-DRA, HLA-DRB1, TNFRSF5, TNFRSF6, TNFSF5, TNFSF6 | 4 | CD4(5), HLA-DRA(3), HLA-DRB1(1) | 334543 | 9 | 8 | 9 | 0 | 5 | 1 | 0 | 3 | 0 | 0 | 0.019 | 0.077 | 1 |
| 5 | FOSBPATHWAY | FOSB gene expression and drug abuse | CDK5, FOSB, GRIA2, JUND, PPP1R1B | 5 | CDK5(1), FOSB(2), GRIA2(14) | 526332 | 17 | 13 | 17 | 4 | 12 | 2 | 1 | 1 | 1 | 0 | 0.087 | 0.079 | 1 |
| 6 | HSA00950_ALKALOID_BIOSYNTHESIS_I | Genes involved in alkaloid biosynthesis I | DDC, GOT1, GOT2, TAT, TYR | 5 | DDC(4), GOT1(3), GOT2(1), TAT(4), TYR(4) | 673736 | 16 | 13 | 16 | 3 | 10 | 2 | 1 | 1 | 2 | 0 | 0.059 | 0.11 | 1 |
| 7 | ASBCELLPATHWAY | B cells require interaction with helper T cells to produce antigen-specific immunoglobulins as a key element of the human immune response. | CD28, CD4, CD80, HLA-DRA, HLA-DRB1, IL10, IL2, IL4, TNFRSF5, TNFRSF6, TNFSF5, TNFSF6 | 8 | CD4(5), CD80(3), HLA-DRA(3), HLA-DRB1(1), IL10(1) | 562026 | 13 | 9 | 13 | 1 | 5 | 1 | 1 | 6 | 0 | 0 | 0.036 | 0.13 | 1 |
| 8 | PLCPATHWAY | Phospholipase C hydrolyzes the membrane lipid PIP2 to DAG, which activates protein kinase C, and IP3, which causes calcium influx. | AKT1, PIK3CA, PIK3R1, PLCB1, PLCG1, PRKCA, PRKCB1, VAV1 | 7 | AKT1(3), PIK3CA(3), PIK3R1(3), PLCB1(16), PLCG1(6), PRKCA(2), VAV1(5) | 1832035 | 38 | 28 | 38 | 6 | 24 | 1 | 2 | 6 | 4 | 1 | 0.0026 | 0.14 | 1 |
| 9 | HSA00472_D_ARGININE_AND_D_ORNITHINE_METABOLISM | Genes involved in D-arginine and D-ornithine metabolism | DAO | 1 | DAO(3) | 102790 | 3 | 3 | 3 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0.54 | 0.16 | 1 |
| 10 | HSA00550_PEPTIDOGLYCAN_BIOSYNTHESIS | Genes involved in peptidoglycan biosynthesis | GLUL, PGLYRP2 | 2 | GLUL(2), PGLYRP2(8) | 260514 | 10 | 6 | 10 | 2 | 6 | 1 | 1 | 2 | 0 | 0 | 0.16 | 0.18 | 1 |
In brief, we tabulate the number of mutations and the number of covered bases for each gene. The counts are broken down by mutation context category: four context categories that are discovered by MutSig, and one for indel and 'null' mutations, which include indels, nonsense mutations, splice-site mutations, and non-stop (read-through) mutations. For each gene, we calculate the probability of seeing the observed constellation of mutations, i.e. the product P1 x P2 x ... x Pm, or a more extreme one, given the background mutation rates calculated across the dataset. [1]
This is an experimental feature. The full results of the analysis summarized in this report can be downloaded from the TCGA Data Coordination Center.